COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis
- PMID: 33571301
- PMCID: PMC7877631
- DOI: 10.1371/journal.pone.0246318
COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis
Abstract
Background: Insight into COVID-19 intensive care unit (ICU) patient characteristics, rates and risks of invasive mechanical ventilation (IMV) and associated outcomes as well as any regional discrepancies is critical in this pandemic for individual case management and overall resource planning.
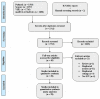
Methods and findings: Electronic searches were performed for reports through May 1 2020 and reports on COVID-19 ICU admissions and outcomes were included using predefined search terms. Relevant data was subsequently extracted and pooled using fixed or random effects meta-analysis depending on heterogeneity. Study quality was assessed by the NIH tool and heterogeneity was assessed by I2 and Q tests. Baseline patient characteristics, ICU and IMV outcomes were pooled and meta-analyzed. Pooled odds ratios (pOR) were calculated for clinical features against ICU, IMV mortality. Subgroup analysis was carried out based on patient regions. A total of twenty-eight studies comprising 12,437 COVID-19 ICU admissions from seven countries were meta-analyzed. Pooled ICU admission rate was 21% [95% CI 0.12-0.34] and 69% of cases needed IMV [95% CI 0.61-0.75]. ICU and IMV mortality were 28.3% [95% CI 0.25-0.32], 43% [95% CI 0.29-0.58] and ICU, IMV duration was 7.78 [95% CI 6.99-8.63] and 10.12 [95% CI 7.08-13.16] days respectively. Besides confirming the significance of comorbidities and clinical findings of COVID-19 previously reported, we found the major correlates with ICU mortality were IMV [pOR 16.46, 95% CI 4.37-61.96], acute kidney injury (AKI) [pOR 12.47, 95% CI 1.52-102.7], and acute respiratory distress syndrome (ARDS) [pOR 6.52, 95% CI 2.66-16.01]. Subgroup analyses confirm significant regional discrepancies in outcomes.
Conclusions: This is a comprehensive systematic review and meta-analysis of COVID-19 ICU and IMV cases and associated outcomes. The significant association of AKI, ARDS and IMV with mortality has implications for ICU resource planning for AKI and ARDS as well as suggesting the need for further research into optimal ventilation strategies for COVID-19 patients in the ICU setting. Regional differences in outcome implies a need to develop region specific protocols for ventilatory support as well as overall treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- National Heart, Lung and Blood Institute (NHLBI). Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

