A role of PIEZO1 in iron metabolism in mice and humans
- PMID: 33571427
- PMCID: PMC7927959
- DOI: 10.1016/j.cell.2021.01.024
A role of PIEZO1 in iron metabolism in mice and humans
Abstract
Iron overload causes progressive organ damage and is associated with arthritis, liver damage, and heart failure. Elevated iron levels are present in 1%-5% of individuals; however, iron overload is undermonitored and underdiagnosed. Genetic factors affecting iron homeostasis are emerging. Individuals with hereditary xerocytosis, a rare disorder with gain-of-function (GOF) mutations in mechanosensitive PIEZO1 ion channel, develop age-onset iron overload. We show that constitutive or macrophage expression of a GOF Piezo1 allele in mice disrupts levels of the iron regulator hepcidin and causes iron overload. We further show that PIEZO1 is a key regulator of macrophage phagocytic activity and subsequent erythrocyte turnover. Strikingly, we find that E756del, a mild GOF PIEZO1 allele present in one-third of individuals of African descent, is strongly associated with increased plasma iron. Our study links macrophage mechanotransduction to iron metabolism and identifies a genetic risk factor for increased iron levels in African Americans.
Keywords: African American; Ion channel; PIEZO1; erythropoiesis; human genetics; iron metabolism; macrophage; mechanotransduction; phagocytosis.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

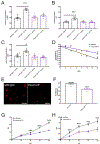
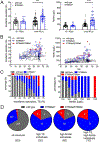
Comment in
-
"Iron"ing out hemophagocytosis through PIEZO1.Cell. 2021 Feb 18;184(4):856-858. doi: 10.1016/j.cell.2021.01.038. Cell. 2021. PMID: 33606984 Free PMC article.
References
-
- Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, et al. (2005). Hemochromatosis and iron-overload screening in a racially diverse population. N. Engl. J. Med 352, 1769–1778. - PubMed
-
- Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Fénéant-Thibault M, Tertian G, de Jaureguiberry J-P, Syfuss P-Y, et al. (2013). Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun 4, 1884. - PMC - PubMed
-
- Andolfo I, Rosato BE, Manna F, De Rosa G, Marra R, Gambale A, Girelli D, Russo R, and Iolascon A (2020). Gain‐of‐function mutations in PIEZO1 directly impair hepatic iron metabolism via the inhibition of the BMP/SMADs pathway. Am J Hematol 95, 188–197. - PubMed
-
- Andrews NC, and Ganz T (2019). The molecular basis of iron metabolism. In Molecular Hematology 4e, Provan D, and Gribben J, eds. (Wiley; ), pp. 161–172.
-
- Aykut B, Chen R, Kim JI, Wu D, Shadaloey SAA, Abengozar R, Preiss P, Saxena A, Pushalkar S, Leinwand J, et al. (2020). Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci. Immunol 5, eabb5168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

