A theoretical study of polymorphism in VQIVYK fibrils
- PMID: 33571490
- PMCID: PMC8105716
- DOI: 10.1016/j.bpj.2021.01.032
A theoretical study of polymorphism in VQIVYK fibrils
Abstract
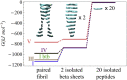
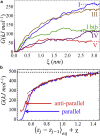
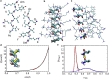
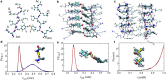
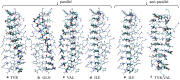
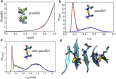
The VQIVYK fragment from the Tau protein, also known as PHF6, is essential for aggregation of Tau into neurofibrillary lesions associated with neurodegenerative diseases. VQIVYK itself forms amyloid fibrils composed of paired β-sheets. Therefore, the full Tau protein and VQIVYK fibrils have been intensively investigated. A central issue in these studies is polymorphism, the ability of a protein to fold into more than one structure. Using all-atom molecular simulations, we generate five stable polymorphs of VQIVYK fibrils, establish their relative free energy with umbrella sampling methods, and identify the side chain interactions that provide stability. The two most stable polymorphs, which have nearly equal free energy, are formed by interdigitation of the mostly hydrophobic VIY "face" sides of the β-sheets. Another stable polymorph is formed by interdigitation of the QVK "back" sides. When we turn to examine structures from cryo-electron microscopy experiments on Tau filaments taken from diseased patients or generated in vitro, we find that the pattern of side chain interactions found in the two most stable face-to-face as well as the back-to-back polymorphs are recapitulated in amyloid structures of the full protein. Thus, our studies suggest that the interactions stabilizing PHF6 fibrils explain the amyloidogenicity of the VQIVYK motif within the full Tau protein and provide justification for the use of VQIVYK fibrils as a test bed for the design of molecules that identify or inhibit amyloid structures.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures







Similar articles
-
Effects of All-Atom Molecular Mechanics Force Fields on Amyloid Peptide Assembly: The Case of PHF6 Peptide of Tau Protein.J Chem Theory Comput. 2021 Oct 12;17(10):6458-6471. doi: 10.1021/acs.jctc.1c00028. Epub 2021 Sep 7. J Chem Theory Comput. 2021. PMID: 34491058 Free PMC article.
-
Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer's disease-like symptoms in animal model.Cell Mol Life Sci. 2020 Jul;77(14):2795-2813. doi: 10.1007/s00018-019-03312-0. Epub 2019 Sep 27. Cell Mol Life Sci. 2020. PMID: 31562564 Free PMC article.
-
Formation and growth of oligomers: a Monte Carlo study of an amyloid tau fragment.PLoS Comput Biol. 2008 Dec;4(12):e1000238. doi: 10.1371/journal.pcbi.1000238. Epub 2008 Dec 5. PLoS Comput Biol. 2008. PMID: 19057640 Free PMC article.
-
Structural Studies of Amyloid Proteins at the Molecular Level.Annu Rev Biochem. 2017 Jun 20;86:69-95. doi: 10.1146/annurev-biochem-061516-045104. Epub 2017 Jan 3. Annu Rev Biochem. 2017. PMID: 28125289 Review.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Mapping the configurational landscape and aggregation phase behavior of the tau protein fragment PHF6.Proc Natl Acad Sci U S A. 2023 Nov 28;120(48):e2309995120. doi: 10.1073/pnas.2309995120. Epub 2023 Nov 20. Proc Natl Acad Sci U S A. 2023. PMID: 37983502 Free PMC article.
-
Shapeshifter W-Tau Peptide Inhibits Tau Aggregation and Disintegrates Paired Helical Filaments.Biochemistry. 2025 Apr 15;64(8):1841-1851. doi: 10.1021/acs.biochem.4c00809. Epub 2025 Mar 26. Biochemistry. 2025. PMID: 40140976 Free PMC article.
-
The SGYS motif of TAF15 prion-like domain is critical to amyloid fibril formation.Biophys J. 2022 Jul 5;121(13):2613-2623. doi: 10.1016/j.bpj.2022.05.038. Epub 2022 May 28. Biophys J. 2022. PMID: 35643629 Free PMC article.
-
Effects of All-Atom Molecular Mechanics Force Fields on Amyloid Peptide Assembly: The Case of PHF6 Peptide of Tau Protein.J Chem Theory Comput. 2021 Oct 12;17(10):6458-6471. doi: 10.1021/acs.jctc.1c00028. Epub 2021 Sep 7. J Chem Theory Comput. 2021. PMID: 34491058 Free PMC article.
-
Investigation of the Structure of Full-Length Tau Proteins with Coarse-Grained and All-Atom Molecular Dynamics Simulations.ACS Chem Neurosci. 2023 Jan 18;14(2):209-217. doi: 10.1021/acschemneuro.2c00381. Epub 2022 Dec 23. ACS Chem Neurosci. 2023. PMID: 36563129 Free PMC article.
References
-
- Knowles T.P.J., Vendruscolo M., Dobson C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014;15:384–396. - PubMed
-
- Knowles T.P.J., Vendruscolo M., Dobson C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014;15:384–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

