Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer's disease
- PMID: 33571811
- PMCID: PMC7928223
- DOI: 10.1016/j.bioorg.2021.104681
Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer's disease
Abstract
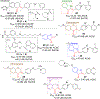
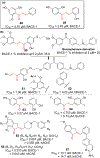
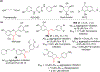
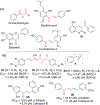
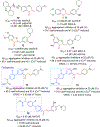
Chalcone [(E)-1,3-diphenyl-2-propene-1-one], a small molecule with α, β unsaturated carbonyl group is a precursor or component of many natural flavonoids and isoflavonoids. It is one of the privileged structures in medicinal chemistry. It possesses a wide range of biological activities encouraging many medicinal chemists to study this scaffold for its usefulness to oncology, infectious diseases, virology and neurodegenerative diseases including Alzheimer's disease (AD). Small molecular size, convenient and cost-effective synthesis, and flexibility for modifications to modulate lipophilicity suitable for blood brain barrier (BBB) permeability make chalcones a preferred candidate for their therapeutic and diagnostic potential in AD. This review summarizes and highlights the importance of chalcone and its analogs as single target small therapeutic agents, multi-target directed ligands (MTDLs) as well as molecular imaging agents for AD. The information summarized here will guide many medicinal chemist and researchers involved in drug discovery to consider chalcone as a potential scaffold for the development of anti-AD agents including theranostics.
Keywords: Alzheimer’s disease; Amyloid beta inhibition; Chalcone; Cholinesterase inhibitors; Molecular probes; Multi-target directed ligands; Neuroinflammation; Theranostic.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures



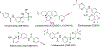
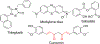



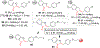
Similar articles
-
Structural Modifications on Chalcone Framework for Developing New Class of Cholinesterase Inhibitors.Int J Mol Sci. 2022 Mar 14;23(6):3121. doi: 10.3390/ijms23063121. Int J Mol Sci. 2022. PMID: 35328542 Free PMC article. Review.
-
Design, synthesis, and evaluation of chalcone-Vitamin E-donepezil hybrids as multi-target-directed ligands for the treatment of Alzheimer's disease.J Enzyme Inhib Med Chem. 2022 Dec;37(1):69-85. doi: 10.1080/14756366.2021.1993845. J Enzyme Inhib Med Chem. 2022. PMID: 34894968 Free PMC article.
-
Design, synthesis, in-silico and biological evaluation of novel chalcone-O-carbamate derivatives as multifunctional agents for the treatment of Alzheimer's disease.Eur J Med Chem. 2019 Sep 15;178:726-739. doi: 10.1016/j.ejmech.2019.06.026. Epub 2019 Jun 14. Eur J Med Chem. 2019. PMID: 31229875
-
Novel donepezil-chalcone-rivastigmine hybrids as potential multifunctional anti-Alzheimer's agents: Design, synthesis, in vitro biological evaluation, in vivo and in silico studies.Bioorg Chem. 2022 Oct;127:106007. doi: 10.1016/j.bioorg.2022.106007. Epub 2022 Jul 7. Bioorg Chem. 2022. PMID: 35849893
-
An ongoing journey of chalcone analogues as single and multi-target ligands in the field of Alzheimer's disease: A review with structural aspects.Life Sci. 2023 May 1;320:121568. doi: 10.1016/j.lfs.2023.121568. Epub 2023 Mar 15. Life Sci. 2023. PMID: 36925061 Review.
Cited by
-
Marine-derived fungi as biocatalysts.Front Microbiol. 2023 Feb 27;14:1125639. doi: 10.3389/fmicb.2023.1125639. eCollection 2023. Front Microbiol. 2023. PMID: 36922968 Free PMC article. Review.
-
Chalcones as Potential Ligands for the Treatment of Parkinson's Disease.Pharmaceuticals (Basel). 2022 Jul 10;15(7):847. doi: 10.3390/ph15070847. Pharmaceuticals (Basel). 2022. PMID: 35890146 Free PMC article. Review.
-
Navigating the treatment landscape of Alzheimer's disease: Current strategies and future directions.Ibrain. 2025 May 10;11(2):162-184. doi: 10.1002/ibra.12197. eCollection 2025 Summer. Ibrain. 2025. PMID: 40546882 Free PMC article. Review.
-
NLRP3 Inflammasome Inhibitors for Antiepileptogenic Drug Discovery and Development.Int J Mol Sci. 2024 May 31;25(11):6078. doi: 10.3390/ijms25116078. Int J Mol Sci. 2024. PMID: 38892264 Free PMC article. Review.
-
In Silico Study in MPO and Molecular Docking of the Synthetic Drynaran Analogues Against the Chronic Tinnitus: Modulation of the M1 Muscarinic Acetylcholine Receptor.Mol Biotechnol. 2024 Feb;66(2):254-269. doi: 10.1007/s12033-023-00748-5. Epub 2023 Apr 20. Mol Biotechnol. 2024. PMID: 37079267
References
-
- Leon-Gonzalez AJ, Acero N, Munoz-Mingarro D, Navarro I, Martin-Cordero C, Chalcones as Promising Lead Compounds on Cancer Therapy, Curr Med Chem, 22 (2015) 3407–3425. - PubMed
-
- Singh P, Anand A, Kumar V, Recent developments in biological activities of chalcones: a mini review, Eur J Med Chem, 85 (2014) 758–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

