Influence of unfused cranial bones on magnetoencephalography signals in human infants
- PMID: 33571879
- PMCID: PMC8012008
- DOI: 10.1016/j.clinph.2020.11.036
Influence of unfused cranial bones on magnetoencephalography signals in human infants
Abstract
Objective: To clarify the effects of unfused cranial bones on magnetoencephalography (MEG) signals during early development.
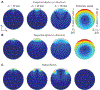
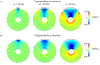
Methods: In a simulation study, we compared the MEG signals over a spherical head model with a circular hole mimicking the anterior fontanel to those over the same head model without the fontanel for different head and fontanel sizes with varying skull thickness and conductivity.
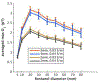
Results: The fontanel had small effects according to three indices. The sum of differences in signal over a sensor array due to a fontanel, for example, was < 6% of the sum without the fontanel. However, the fontanel effects were extensive for dipole sources deep in the brain or outside the fontanel for larger fontanels. The effects were comparable in magnitude for tangential and radial sources. Skull thickness significantly increased the effect, while skull conductivity had minor effects.
Conclusion: MEG signal is weakly affected by a fontanel. However, the effects can be extensive and significant for radial sources, thicker skull and large fontanels. The fontanel effects can be intuitively explained by the concept of secondary sources at the fontanel wall.
Significance: The minor influence of unfused cranial bones simplifies MEG analysis, but it should be considered for quantitative analysis.
Keywords: Brain development; Electroencephalography (EEG); Fontanel; Human brain mapping; Magnetoencephalography (MEG); Unfused cranial bones.
Copyright © 2021 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



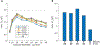


References
-
- Barth DS, Sutherling W, Broffman J, Beatty J. Magnetic localization of a dipolar current source implanted in a sphere and a human cranium. Electroencephalogr Clin Neurophysiol. 1986;63:260–73. - PubMed
-
- Cohen D, Cuffin BN. Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol. 1983;56:38–51. - PubMed
-
- Cohen MM Jr. Sutural biology and the correlates of craniosynostosis. Am J Med Genet. 1993;47:581–616. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

