PEG-Coated Large Mesoporous Silicas as Smart Platform for Protein Delivery and Their Use in a Collagen-Based Formulation for 3D Printing
- PMID: 33572076
- PMCID: PMC7914545
- DOI: 10.3390/ijms22041718
PEG-Coated Large Mesoporous Silicas as Smart Platform for Protein Delivery and Their Use in a Collagen-Based Formulation for 3D Printing
Abstract
Silica-based mesoporous systems have gained great interest in drug delivery applications due to their excellent biocompatibility and high loading capability. However, these materials face challenges in terms of pore-size limitations since they are characterized by nanopores ranging between 6-8 nm and thus unsuitable to host large molecular weight molecules such as proteins, enzymes and growth factors (GFs). In this work, for an application in the field of bone regeneration, large-pore mesoporous silicas (LPMSs) were developed to vehicle large biomolecules and release them under a pH stimulus. Considering bone remodeling, the proposed pH-triggered mechanism aims to mimic the release of GFs encased in the bone matrix due to bone resorption by osteoclasts (OCs) and the associated pH drop. To this aim, LPMSs were prepared by using 1,3,5-trimethyl benzene (TMB) as a swelling agent and the synthesis solution was hydrothermally treated and the influence of different process temperatures and durations on the resulting mesostructure was investigated. The synthesized particles exhibited a cage-like mesoporous structure with accessible pores of diameter up to 23 nm. LPMSs produced at 140 °C for 24 h showed the best compromise in terms of specific surface area, pores size and shape and hence, were selected for further experiments. Horseradish peroxidase (HRP) was used as model protein to evaluate the ability of the LPMSs to adsorb and release large biomolecules. After HRP-loading, LPMSs were coated with a pH-responsive polymer, poly(ethylene glycol) (PEG), allowing the release of the incorporated biomolecules in response to a pH decrease, in an attempt to mimic GFs release in bone under the acidic pH generated by the resorption activity of OCs. The reported results proved that PEG-coated carriers released HRP more quickly in an acidic environment, due to the protonation of PEG at low pH that catalyzes polymer hydrolysis reaction. Our findings indicate that LPMSs could be used as carriers to deliver large biomolecules and prove the effectiveness of PEG as pH-responsive coating. Finally, as proof of concept, a collagen-based suspension was obtained by incorporating PEG-coated LPMS carriers into a type I collagen matrix with the aim of designing a hybrid formulation for 3D-printing of bone scaffolds.
Keywords: 3D printing; growth factor; hydrothermal treatment; large pores; mesoporous silica particles; pH-triggered release; type I collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



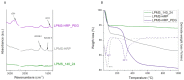
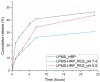



Similar articles
-
Unsaturated nitrogen-rich polymer poly(l-histidine) gated reversibly switchable mesoporous silica nanoparticles using "graft to" strategy for drug controlled release.Acta Biomater. 2017 Nov;63:150-162. doi: 10.1016/j.actbio.2017.08.050. Epub 2017 Sep 2. Acta Biomater. 2017. PMID: 28873341
-
Thermo- and pH-responsive targeted lipid-coated mesoporous nano silica platform for dual delivery of paclitaxel and gemcitabine to overcome HER2-positive breast cancer.Int J Pharm. 2023 Dec 15;648:123606. doi: 10.1016/j.ijpharm.2023.123606. Epub 2023 Nov 14. Int J Pharm. 2023. PMID: 37972671
-
Intracellular pH-Triggered, Targeted Drug Delivery to Cancer Cells by Multifunctional Envelope-Type Mesoporous Silica Nanocontainers.ACS Appl Mater Interfaces. 2015 Aug 12;7(31):17399-407. doi: 10.1021/acsami.5b04684. Epub 2015 Jul 30. ACS Appl Mater Interfaces. 2015. PMID: 26196506
-
Chemoresponsive smart mesoporous silica systems - An emerging paradigm for cancer therapy.Int J Pharm. 2018 Dec 20;553(1-2):310-326. doi: 10.1016/j.ijpharm.2018.10.026. Epub 2018 Oct 10. Int J Pharm. 2018. PMID: 30316004 Review.
-
Drug release from ordered mesoporous silicas.Curr Pharm Des. 2015;21(42):6213-819. doi: 10.2174/1381612822666151106121419. Curr Pharm Des. 2015. PMID: 26549760 Review.
Cited by
-
The Physical Chemistry and Chemical Physics (PCCP) Section of the International Journal of Molecular Sciences in Its Publications: The First 300 Thematic Articles in the First 3 Years.Int J Mol Sci. 2021 Dec 27;23(1):241. doi: 10.3390/ijms23010241. Int J Mol Sci. 2021. PMID: 35008667 Free PMC article.
-
Advantages of nanoencapsulation in the delivery of therapeutics for bone regeneration.Nanomedicine (Lond). 2025 Apr;20(8):773-775. doi: 10.1080/17435889.2025.2462518. Epub 2025 Feb 5. Nanomedicine (Lond). 2025. PMID: 39907195 No abstract available.
-
Photopolymerization strategy for the preparation of small-diameter artificial blood vessels with micro-nano structures on the inner wall.Biomed Opt Express. 2021 Aug 26;12(9):5844-5854. doi: 10.1364/BOE.432441. eCollection 2021 Sep 1. Biomed Opt Express. 2021. PMID: 34692219 Free PMC article.
-
Optimization of an Injectable, Resorbable, Bioactive Cement Able to Release the Anti-Osteoclastogenic Biomolecule ICOS-Fc for the Treatment of Osteoporotic Vertebral Compression Fractures.Biomolecules. 2023 Jan 2;13(1):94. doi: 10.3390/biom13010094. Biomolecules. 2023. PMID: 36671479 Free PMC article.
-
Smart Bioinks for the Printing of Human Tissue Models.Biomolecules. 2022 Jan 15;12(1):141. doi: 10.3390/biom12010141. Biomolecules. 2022. PMID: 35053289 Free PMC article. Review.
References
-
- Witharana C., Wanigasekara J. Drug Delivery Systems: A New Frontier in Nano-Technology. Int. J. Med. Res. Health Sci. 2017;6:11–14.
-
- Shi Z., Zhou Y., Fan T., Lin Y., Zhang H., Mei L. Inorganic Nano-Carriers Based Smart Drug Delivery Systems for Tumor Therapy. Smart Mater. Med. 2020;1:32–47. doi: 10.1016/j.smaim.2020.05.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

