Silencing ZEB2 Induces Apoptosis and Reduces Viability in Glioblastoma Cell Lines
- PMID: 33572092
- PMCID: PMC7916008
- DOI: 10.3390/molecules26040901
Silencing ZEB2 Induces Apoptosis and Reduces Viability in Glioblastoma Cell Lines
Abstract
Background: Glioma is an aggressive type of brain tumor that originated from neuroglia cells, accounts for about 80% of all malignant brain tumors. Glioma aggressiveness has been associated with extreme cell proliferation, invasion of malignant cells, and resistance to chemotherapies. Due to resistance to common therapies, glioma affected patients' survival has not been remarkably improved. ZEB2 (SIP1) is a critical transcriptional regulator with various functions during embryonic development and wound healing that has abnormal expression in different malignancies, including brain tumors. ZEB2 overexpression in brain tumors is attributed to an unfavorable state of the malignancy. Therefore, we aimed to investigate some functions of ZEB2 in two different glioblastoma U87 and U373 cell lines.
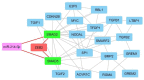
Methods: In this study, we investigated the effect of ZEB2 knocking down on the apoptosis, cell cycle, cytotoxicity, scratch test of the two malignant brain tumor cell lines U87 and U373. Besides, we investigated possible proteins and microRNA, SMAD2, SMAD5, and miR-214, which interact with ZEB2 via in situ analysis. Then we evaluated candidate gene expression after ZEB2-specific knocking down.
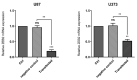
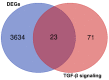
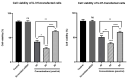
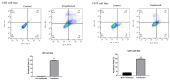
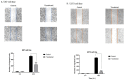
Results: We found that ZEB2 suppression induced apoptosis in U87 and U373 cell lines. Besides, it had cytotoxic effects on both cell lines and reduced cell migration. Cell cycle analysis showed cell cycle arrest in G0/G1 and apoptosis induction in U87 and U373 cell lines receptively. Also, we have found that SAMAD2/5 expression was reduced after ZEB2-siRNA transfection and miR-214 upregulated after transfection.
Conclusions: In line with previous investigations, our results indicated a critical oncogenic role for ZEB2 overexpression in brain glioma tumors. These properties make ZEB2 an essential molecule for further studies in the treatment of glioma cancer.
Keywords: TGF-β pathway; ZEB2; apoptosis; glioblastoma; siRNA.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Role of ZEB2 Expression in Pediatric and Adult Glioblastomas.Anticancer Res. 2021 Jan;41(1):175-185. doi: 10.21873/anticanres.14763. Anticancer Res. 2021. PMID: 33419811
-
ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma.PLoS One. 2012;7(6):e38842. doi: 10.1371/journal.pone.0038842. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22761708 Free PMC article.
-
Long non-coding RNA HOTAIRM1 promotes proliferation and inhibits apoptosis of glioma cells by regulating the miR-873-5p/ZEB2 axis.Chin Med J (Engl). 2020 Jan 20;133(2):174-182. doi: 10.1097/CM9.0000000000000615. Chin Med J (Engl). 2020. PMID: 31929367 Free PMC article.
-
Targeting strategies on miRNA-21 and PDCD4 for glioblastoma.Arch Biochem Biophys. 2015 Aug 15;580:64-74. doi: 10.1016/j.abb.2015.07.001. Epub 2015 Jul 2. Arch Biochem Biophys. 2015. PMID: 26142886 Review.
-
Potential of microRNA based diagnostics and therapeutics in glioma: a patent review.Expert Opin Ther Pat. 2021 Jan;31(1):91-106. doi: 10.1080/13543776.2021.1837775. Epub 2020 Dec 10. Expert Opin Ther Pat. 2021. PMID: 33054467 Review.
Cited by
-
EphrinB2-EphB4 Signaling in Neurooncological Disease.Int J Mol Sci. 2022 Jan 31;23(3):1679. doi: 10.3390/ijms23031679. Int J Mol Sci. 2022. PMID: 35163601 Free PMC article. Review.
-
The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications.Cancers (Basel). 2022 Feb 14;14(4):940. doi: 10.3390/cancers14040940. Cancers (Basel). 2022. PMID: 35205692 Free PMC article. Review.
-
In Vitro and Computational Studies of Perezone and Perezone Angelate as Potential Anti-Glioblastoma Multiforme Agents.Molecules. 2022 Feb 26;27(5):1565. doi: 10.3390/molecules27051565. Molecules. 2022. PMID: 35268667 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

