Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis
- PMID: 33572149
- PMCID: PMC7915816
- DOI: 10.3390/cancers13040695
Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis
Abstract
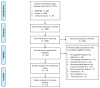
The role of cytoreductive nephrectomy (CN) in the treatment of metastatic renal cell carcinoma (mRCC) remains controversial during the targeted therapy era. To reconcile the current literature, we analyzed the reported survival data at the individual patient level and compared the long-term survival outcomes of CN combined with targeted therapy vs. targeted therapy alone in patients with mRCC. We performed a systematic review of the literature using the MEDLINE, Scopus, and Cochrane Library databases (end-of-search date: 21 July 2020). We recuperated individual patient data from the Kaplan-Meier curves for overall (OS), progression-free (PFS), and cancer-specific survival (CSS) from each study. We subsequently performed one-stage frequentist and Bayesian random-effects meta-analyses using both Cox proportional hazards and restricted mean survival time (RMST) models. Two-stage random-effects meta-analyses were also performed as sensitivity analyses. A subgroup analysis was also performed to determine the effect of CN timing. Fifteen studies fulfilling our inclusion criteria were identified, including fourteen retrospective cohort studies and one randomized controlled trial. In the one-stage frequentist meta-analysis, the CN group had superior OS (hazard ratio [HR]: 0.58, 95% confidence interval [CI]: 0.54-0.62, p < 0.0001) and CSS (HR: 0.63, 95% CI: 0.53-0.75, p < 0.0001). No meaningful clinical difference was observed in PFS (HR: 0.90, 95% CI: 0.80-1.02, p = 0.09). One-stage Bayesian meta-analysis also revealed superior OS (HR: 0.59, 95% credibility interval [CrI]: 0.55-0.63) and CSS (HR: 0.63, 95% CrI: 0.53-0.75) in the CN group, while no meaningful clinical difference was detected in PFS (HR: 0.91, 95% CrI: 0.80-1.02). Similar results were obtained with the RMST models. The OS benefit was also noted in the two-stage meta-analyses models, and in the subgroup of patients who received upfront CN. The combination of CN and targeted therapy for mRCC may lead to superior long-term survival outcomes compared to targeted therapy alone. Careful patient selection based on prognostic factors is required to optimize outcomes.
Keywords: VEGF inhibitors; cytoreductive nephrectomy; mammalian target of rapamycin inhibitors; renal cell carcinoma; targeted therapy; tyrosine kinase inhibitors.
Conflict of interest statement
P.M. has received honoraria for service on a Scientific Advisory Board for Mirati Therapeutics, BMS, and Exelixis; consulting for Axiom Healthcare Strategies; non-branded educational programs supported by Exelixis and Pfizer; and research funding for clinical trials from Takeda, BMS, Mirati Therapeutics, Gateway for Cancer Research, and UT MD Anderson Cancer Center. N.M.T. has received honoraria for service on Scientific Advisory Boards for Bristol-Myers Squibb, Eli Lilly and Company, Exelixis, Inc., and Nektar Therapeutics; for strategic council meetings with Eisai Inc.; steering committee meetings with Pfizer, Inc.; and for seminar presentations for Ono Pharmaceutical Co., Ltd.; as well as research funding for clinical trials from Exelixis, Inc., Calithera Biosciences, and Nektar. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

