Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age
- PMID: 33572157
- PMCID: PMC7915891
- DOI: 10.3390/ijms22041725
Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age
Abstract
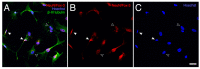
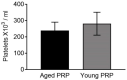
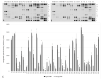
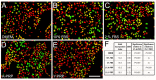
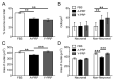
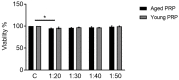
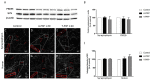
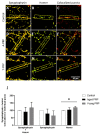
Platelet-rich plasma (PRP) is a biologic therapy that promotes healing responses across multiple medical fields, including the central nervous system (CNS). The efficacy of this therapy depends on several factors such as the donor's health status and age. This work aims to prove the effect of PRP on cellular models of the CNS, considering the differences between PRP from young and elderly donors. Two different PRP pools were prepared from donors 65‒85 and 20‒25 years old. The cellular and molecular composition of both PRPs were analyzed. Subsequently, the cellular response was evaluated in CNS in vitro models, studying proliferation, neurogenesis, synaptogenesis, and inflammation. While no differences in the cellular composition of PRPs were found, the molecular composition of the Young PRP showed lower levels of inflammatory molecules such as CCL-11, as well as the presence of other factors not found in Aged PRP (GDF-11). Although both PRPs had effects in terms of reducing neural progenitor cell apoptosis, stabilizing neuronal synapses, and decreasing inflammation in the microglia, the effect of the Young PRP was more pronounced. In conclusion, the molecular composition of the PRP, conditioned by the age of the donors, affects the magnitude of the biological response.
Keywords: aging; central nervous system; growth factors; microglia; neurons; platelet-rich plasma.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Anitua E., Pascual C., Pérez-Gonzalez R., Antequera D., Padilla S., Orive G., Carro E. Intranasal delivery of plasma and platelet growth factors using prgf-endoret system enhances neurogenesis in a mouse model of Alzheimer’s disease. PLoS ONE. 2013;8:e73118. doi: 10.1371/journal.pone.0073118. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

