Porcine Circovirus 3 Detection in Aborted Fetuses and Stillborn Piglets from Swine Reproductive Failure Cases
- PMID: 33572209
- PMCID: PMC7915229
- DOI: 10.3390/v13020264
Porcine Circovirus 3 Detection in Aborted Fetuses and Stillborn Piglets from Swine Reproductive Failure Cases
Abstract
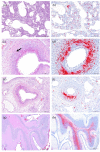
Porcine circovirus 3 (PCV-3) has been widely detected in healthy and diseased pigs; among different pathologic conditions, the strongest evidence of association comes from reproductive disease cases. However, simple viral detection does not imply the causality of the clinical conditions. Detection of PCV-3 within lesions may provide stronger evidence of causality. Thus, this study aimed to assess the frequency of PCV-3 detection in tissues from fetuses/stillborn piglets in cases of reproductive problems in domestic swine, as well as the histopathologic assessment of fetal tissues. Fetuses or stillborn piglets from 53 cases of reproductive failure were collected and analyzed by PCV-3 qPCR. The presence of porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus 2 (PCV-2), and porcine parvovirus 1 (PPV1) was also checked. PCV-3 qPCR positive samples with a high viral load were tested by PCV-3 in situ hybridization (ISH), sequenced, and phylogenetically analyzed. PCV-3 DNA was detected in 18/53 (33.9%) reproductive failure cases and in 16 of them PCV-3 was the only pathogen found. PCV-2 DNA was found in 5/53 (9.4%), PRRSV RNA in 4/53 (7.5%) and PPV1 was not detected. Four out of the six PCV-3 qPCR-positive cases with Ct value <30 were positive when tested by ISH. In these samples, PCV-3 was detected within mild histopathologic lesions, such as arteritis and periarteritis in multiple tissues. The present work emphasizes the need to include PCV-3 as a potential causative agent of reproductive failure in swine.
Keywords: aborted fetuses; histopathology; in situ hybridization; porcine circovirus 3 (PCV-3); quantitative PCR; reproductive failure; stillborn.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Althouse G.C., Kauffold J., Rossow S. Diseases of the reproductive system. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., Zhang. J., editors. Diseases of Swine. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2019. pp. 373–392.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

