Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial
- PMID: 33572213
- PMCID: PMC7915437
- DOI: 10.3390/jcm10040662
Changes in Plasma Glial Fibrillary Acidic Protein in Children Receiving Sevoflurane Anesthesia: A Preliminary Randomized Trial
Abstract
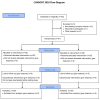
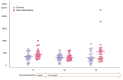
We investigated changes in plasma glial fibrillary acidic protein concentration during sevoflurane anesthesia induction in children < 3 years old and determined the effect of co-administering dexmedetomidine. This preliminary randomized trial included 60 pediatric patients who received sevoflurane anesthesia for >3 h. Patients were assigned to dexmedetomidine or control groups at a 1:1 ratio. The primary outcome was changes in plasma glial fibrillary acidic protein concentration of dexmedetomidine and control groups over time. Fifty-five patients were included in the final analysis. The median (interquartile range (IQR)) of the plasma glial fibrillary acidic protein level was 387.7 (298.9-510.8) pg·mL-1 immediately after anesthetic induction, 302.6 (250.9-412.5) pg·mL-1 at 30 min, and 321.9 (233.8-576.2) pg·mL-1 at 180 min after the first sample. These values did not change over time (p = 0.759). However, plasma glial fibrillary acidic protein increased after 180 min of infusion of dexmedetomidine compared with values at 30 min infusion (p = 0.04, mean difference and 95% confidence interval of 221.6 and 2.2 to 441.0 pg·mL-1). In conclusion, three hours of sevoflurane anesthesia in pediatric patients < 3 years old did not provoke neuronal injury assessed by the plasma biomarker. Further studies regarding the effect of prolonged dexmedetomidine infusion on anesthetic neuronal injury are required.
Keywords: dexmedetomidine; general anesthesia; glial fibrillary acidic protein; pediatrics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation.Pediatr Crit Care Med. 2011 Sep;12(5):572-9. doi: 10.1097/PCC.0b013e3181fe3ec7. Pediatr Crit Care Med. 2011. PMID: 21057367 Free PMC article.
-
[Effects of different maintain doses of dexmedetomidine on plasma cortisol and glucose during anesthesia recovery period in patients undergoing uvulopalatopharyngoplasty under sevoflurane inhalation anesthesia].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014 Aug;28(15):1154-7. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014. PMID: 25322607 Clinical Trial. Chinese.
-
Optimal Dexmedetomidine Dose to Prevent Emergence Agitation Under Sevoflurane and Remifentanil Anesthesia During Pediatric Tonsillectomy and Adenoidectomy.Front Pharmacol. 2019 Sep 19;10:1091. doi: 10.3389/fphar.2019.01091. eCollection 2019. Front Pharmacol. 2019. PMID: 31607927 Free PMC article.
-
Effects of dexmedetomidine on sevoflurane requirement for 50% excellent tracheal intubation in children: a randomized, double-blind comparison.Paediatr Anaesth. 2014 Sep;24(9):987-93. doi: 10.1111/pan.12430. Epub 2014 May 14. Paediatr Anaesth. 2014. PMID: 24823715 Review.
-
Effect of dexmedetomidine in children undergoing general anesthesia with sevoflurane: a meta-analysis.Braz J Anesthesiol. 2017 Mar-Apr;67(2):193-198. doi: 10.1016/j.bjane.2016.02.007. Epub 2016 Nov 25. Braz J Anesthesiol. 2017. PMID: 28236868 Review.
Cited by
-
Comparison of etomidate and propofol-mediated anesthesia induction followed by intubation and sevoflurane maintenance during ERCP in obese patients.Am J Transl Res. 2021 Aug 15;13(8):9853-9859. eCollection 2021. Am J Transl Res. 2021. PMID: 34540121 Free PMC article.
References
-
- DeFrances C.J., Cullen K.A., Kozak L.J. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13:1–209. - PubMed
-
- US Food and Drug Administration Evaluation of Automatic Class III Designation for Banyan Brain Trauma Indicator. [(accessed on 1 July 2019)]; Available online: https://www. accessdata.fda.gov/cdrh_docs/reviews/DEN170045.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources