Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.)
- PMID: 33572261
- PMCID: PMC7915531
- DOI: 10.3390/molecules26040914
Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.)
Abstract
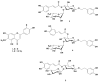
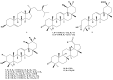
Chromatographic purification of the alcoholic extract from the aerial parts of the Saudi plant Nuxia oppositifolia (Hochst.), Benth., resulted in five isolated phenolic compounds. Two flavones, hispidulin (1) and jaceosidin (2), and the phenylethanoid glycosides, verbascoside (3), isoverbascoside (4), and conandroside (5), were identified and their chemical structures were determined by spectroscopic analyses. The insecticidal activity of compounds 1 and 2, in addition to 11 compounds isolated in a previous research (6-16), was evaluated against the Yellow Fever mosquito, Aedes aegypti. Four compounds displayed adulticidal activity with LD50 values of 2-2.3 μg/mosquito. Free radical scavenging properties of the plant extracts and compounds (1-5) were evaluated by measuring the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonate radical cation (ABTS•+) scavenging activity. All compounds exhibited notable activity, compared with the positive control, l-Ascorbic acid. This study suggests that N. oppositifolia could be a promising source of secondary metabolites, some with lethal adulticidal effect against Ae. aegypti.
Keywords: ABTS; Aedes aegypti; DPPH; Nuxia oppositifolia; biopesticides; flavonoids; free radical scavenging; mosquito control; phenylethanoid; triterpenes.
Conflict of interest statement
The authors declare no conflict of interest. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Figures
Similar articles
-
Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia.Biomolecules. 2019 Dec 30;10(1):61. doi: 10.3390/biom10010061. Biomolecules. 2019. PMID: 31905962 Free PMC article.
-
New Cytotoxic Seco-Type Triterpene and Labdane-Type Diterpenes from Nuxia oppositifolia.Molecules. 2017 Mar 2;22(3):389. doi: 10.3390/molecules22030389. Molecules. 2017. PMID: 28257105 Free PMC article.
-
Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts.Molecules. 2020 Nov 25;25(23):5537. doi: 10.3390/molecules25235537. Molecules. 2020. PMID: 33255853 Free PMC article.
-
Chemical Composition of Buddleja polystachya Aerial Parts and its Bioactivity against Aedes aegypti.Nat Prod Res. 2018 Dec;32(23):2775-2782. doi: 10.1080/14786419.2017.1378213. Epub 2017 Sep 25. Nat Prod Res. 2018. PMID: 28942684
-
Larvicidal activity of Ottonia anisum metabolites against Aedes aegypti: A potential natural alternative source for mosquito vector control in Brazil.J Vector Borne Dis. 2017 Jan-Mar;54(1):61-68. J Vector Borne Dis. 2017. PMID: 28352047
Cited by
-
A Themed Issue in Honor of Professor K. Hüsnü Can Baser-Outstanding Contributions in the Fields of Pharmacognosy, Phytochemistry, Botany and Ethnopharmacology.Molecules. 2021 Sep 10;26(18):5507. doi: 10.3390/molecules26185507. Molecules. 2021. PMID: 34576976 Free PMC article.
-
Saudi Arabian Plants: A Powerful Weapon against a Plethora of Diseases.Plants (Basel). 2022 Dec 8;11(24):3436. doi: 10.3390/plants11243436. Plants (Basel). 2022. PMID: 36559548 Free PMC article. Review.
-
The Pharmacological Spectrum of Hispidulin: A Thorough Systematic Review and Meta-analysis.Cell Biochem Biophys. 2025 Aug 21. doi: 10.1007/s12013-025-01871-7. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40839205 Review. No abstract available.
References
-
- Jensen S.R., Ravnkilde L., Schripsema J. Unedoside derivatives in Nuxia and their biosynthesis. Phytochemistry. 1998;47:1007–1011. doi: 10.1016/S0031-9422(98)80062-9. - DOI
-
- Jensen S.R. Chemotaxonomy of the genus Nuxia (Buddlejaceae) Stud. Plant Sci. 1999;6:379–382.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous