EphA2 and EGFR: Friends in Life, Partners in Crime. Can EphA2 Be a Predictive Biomarker of Response to Anti-EGFR Agents?
- PMID: 33572284
- PMCID: PMC7915460
- DOI: 10.3390/cancers13040700
EphA2 and EGFR: Friends in Life, Partners in Crime. Can EphA2 Be a Predictive Biomarker of Response to Anti-EGFR Agents?
Abstract
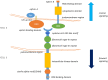
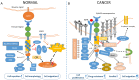
The Eph receptors represent the largest group among Receptor Tyrosine kinase (RTK) families. The Eph/ephrin signaling axis plays center stage during development, and the deep perturbation of signaling consequent to its dysregulation in cancer reveals the multiplicity and complexity underlying its function. In the last decades, they have emerged as key players in solid tumors, including colorectal cancer (CRC); however, what causes EphA2 to switch between tumor-suppressive and tumor-promoting function is still an active theater of investigation. This review summarizes the recent advances in understanding EphA2 function in cancer, with detail on the molecular determinants of the oncogene-tumor suppressor switch function of EphA2. We describe tumor context-specific examples of EphA2 signaling and the emerging role EphA2 plays in supporting cancer-stem-cell-like populations and overcoming therapy-induced stress. In such a frame, we detail the interaction of the EphA2 and EGFR pathway in solid tumors, including colorectal cancer. We discuss the contribution of the EphA2 oncogenic signaling to the resistance to EGFR blocking agents, including cetuximab and TKIs.
Keywords: CRC; CSCs; EGFR; EphA2; TKI; cetuximab; drug resistance; ephrins; inter-tumor heterogeneity; intra-tumor heterogeneity.
Conflict of interest statement
No potential conflict of interest were disclosed.
Figures

References
-
- Himanen J.P., Goldgur Y., Miao H., Myshkin E., Guo H., Buck M., Nguyen M., Rajashankar K.R., Wang B., Nikolov D.B. Ligand recognition by A-class Eph receptors: Crystal structures of the EphA2 ligand-binding domain and the EphA2/ephrin-A1 complex. EMBO Rep. 2009;10:722–728. doi: 10.1038/embor.2009.91. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

