Recent Advances in Quenchbody, a Fluorescent Immunosensor
- PMID: 33572319
- PMCID: PMC7916128
- DOI: 10.3390/s21041223
Recent Advances in Quenchbody, a Fluorescent Immunosensor
Abstract
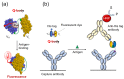
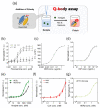
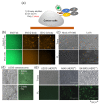
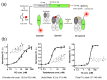
The detection of viruses, disease biomarkers, physiologically active substances, drugs, and chemicals is of great significance in many areas of our lives. Immunodetection technology is based on the specificity and affinity of antigen-antibody reactions. Compared with other analytical methods such as liquid chromatography coupled with mass spectrometry, which requires a large and expensive instrument, immunodetection has the advantages of simplicity and good selectivity and is thus widely used in disease diagnosis and food/environmental monitoring. Quenchbody (Q-body), a new type of fluorescent immunosensor, is an antibody fragment labeled with fluorescent dyes. When the Q-body binds to its antigen, the fluorescence intensity increases. The detection of antigens by changes in fluorescence intensity is simple, easy to operate, and highly sensitive. This review comprehensively discusses the principle, construction, application, and current progress related to Q-bodies.
Keywords: antibody; biomarker; detection; fluorescence; immunoassay; quench.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- de Picciotto S., Dickson P.M., Traxlmayr M.W., Marques B.S., Socher E., Zhao S., Cheung S., Kiefer J.D., Wand A.J., Griffith L.G., et al. Design Principles for SuCESsFul Biosensors: Specific Fluorophore/Analyte Binding and Minimization of Fluorophore/Scaffold Interactions. J. Mol. Biol. 2016;428:4228–4241. doi: 10.1016/j.jmb.2016.07.004. - DOI - PMC - PubMed
-
- Islam J., Riley B.T., Fercher C., Jones M.L., Buckle A.M., Howard C.B., Cox R.P., Bell T.D.M., Mahler S., Corrie S.R. Wavelength-Dependent Fluorescent Immunosensors via Incorporation of Polarity Indicators near the Binding Interface of Antibody Fragments. Anal. Chem. 2019;91:7631–7638. doi: 10.1021/acs.analchem.9b00445. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

