Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis
- PMID: 33572320
- PMCID: PMC7916132
- DOI: 10.3390/ijms22041742
Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis
Abstract
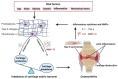
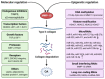
Osteoarthritis (OA) is a common degenerative disease characterized by the destruction of articular cartilage and chronic inflammation of surrounding tissues. Matrix metalloproteinase-13 (MMP-13) is the primary MMP involved in cartilage degradation through its particular ability to cleave type II collagen. Hence, it is an attractive target for the treatment of OA. However, the detailed molecular mechanisms of OA initiation and progression remain elusive, and, currently, there are no interventions available to restore degraded cartilage. This review fully illustrates the involvement of MMP-13 in the initiation and progression of OA through the regulation of MMP-13 activity at the molecular and epigenetic levels, as well as the strategies that have been employed against MMP-13. The aim of this review is to identify MMP-13 as an attractive target for inhibitor development in the treatment of OA.
Keywords: MMP-13; cartilage; inhibitor; matrix metalloproteinases; osteoarthritis; regulation; type II collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Castrogiovanni P., Musumeci G. Which is the best physical treatment for osteoarthritis? J. Funct. Morphol. Kinesiol. 2016;1:54–68. doi: 10.3390/jfmk1010054. - DOI
-
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

