Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches
- PMID: 33572373
- PMCID: PMC7916193
- DOI: 10.3390/biomedicines9020171
Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches
Abstract
Cutaneous squamous cell carcinoma (cSCC), a non-melanoma skin cancer, is a keratinocyte carcinoma representing one of the most common cancers with an increasing incidence. cSCC could be in situ (e.g., Bowen's disease) or an invasive form. A significant cSCC risk factor is advanced age, together with cumulative sun exposure, fair skin, prolonged immunosuppression, and previous skin cancer diagnoses. Although most cSCCs can be treated by surgery, a fraction of them recur and metastasize, leading to death. cSCC could arise de novo or be the result of a progression of the actinic keratosis, an in situ carcinoma. The multistage process of cSCC development and progression is characterized by mutations in the genes involved in epidermal homeostasis and by several alterations, such as epigenetic modifications, viral infections, or microenvironmental changes. Thus, cSCC development is a gradual process with several histological- and pathological-defined stages. Dermoscopy and reflectance confocal microscopy enhanced the diagnostic accuracy of cSCC. Surgical excision is the first-line treatment for invasive cSCC. Moreover, radiotherapy may be considered as a primary treatment in patients not candidates for surgery. Extensive studies of cSCC pathogenic mechanisms identified several pharmaceutical targets and allowed the development of new systemic therapies, including immunotherapy with immune checkpoint inhibitors, such as Cemiplimab, and epidermal growth factor receptor inhibitors for metastatic and locally advanced cSCC. Furthermore, the implementation of prevention measures has been useful in patient management.
Keywords: Bowen’s disease; cemiplimab; dermoscopy; immunotherapy; keratinocyte carcinoma; non-melanoma skin cancer; radiotherapy; squamous cell carcinoma; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
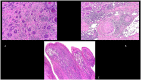
Figures





References
-
- Martincorena I., Roshan A., Gerstung M., Ellis P., van Loo P., McLaren S., Wedge D.C., Fullam A., Alexandrov L.B., Tubio J.M., et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. - DOI - PMC - PubMed
-
- Skulsky S.L., O’Sullivan B., McArdle O., Leader M., Roche M., Conlon P.J., O’Neill J.P. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines In Oncology. Head Neck. 2017;39:578–594. doi: 10.1002/hed.24580. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

