Protective Effects of Kirenol against Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of the Proinflammatory NFκB Pathway and the AMPK2-/Nrf2-Mediated HO-1/AOE Pathway
- PMID: 33572510
- PMCID: PMC7911485
- DOI: 10.3390/antiox10020204
Protective Effects of Kirenol against Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of the Proinflammatory NFκB Pathway and the AMPK2-/Nrf2-Mediated HO-1/AOE Pathway
Abstract
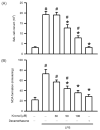
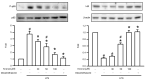
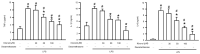
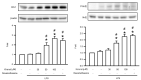
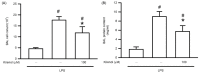
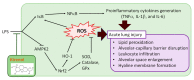
Acute lung injury (ALI) is an acute and life-threatening inflammatory disease of the lung parenchyma that is associated with high mortality worldwide. No therapeutic strategies have been developed for the mitigation of the proinflammatory response that characterizes ALI. Kirenol has anti-inflammatory, antiarthritic, and immunoregulatory effects. In the present study, we investigated the protective effects of kirenol against lipopolysaccharides (LPS)-induced ALI in mice. Kirenol reduced the LPS-induced histopathology changes involving edema and thickening of the interstitial or alveolar walls, infiltration of leukocytes, formation of hyaline membrane. Pretreatment with kirenol reduced leukocytes infiltration in bronchoalveolar lavage fluid (BALF), the alveolar-capillary barrier disruption and lipid peroxidation in lung tissues induced by LPS. Kirenol significantly inhibited the secretion of cytokines, IL-1β, IL6, and TNFα, into the BALF of the mice with LPS-induced ALI through NFκB activation. Moreover, kirenol attenuated the downregulation of the antioxidant enzymes, superoxide dismutase, glutathione peroxidase, and catalase that was induced by LPS. HO-1 expression and the phosphorylation of Nrf2 and AMPK2 were also induced by kirenol. The results indicate that kirenol can be developed as a treatment strategy for ALI, and its effects are induced through the inhibition of the NF-κB proinflammatory pathway and promotion of AMPK2/Nrf2-mediated HO-1 and antioxidant enzymes (AOE) activation.
Keywords: AMPK2/Nrf2-mediated HO-1; AOE pathway; NF-κB pathway; acute lung injury; kirenol; lipopolysaccharide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Downregulated microRNA-27b attenuates lipopolysaccharide-induced acute lung injury via activation of NF-E2-related factor 2 and inhibition of nuclear factor κB signaling pathway.J Cell Physiol. 2019 May;234(5):6023-6032. doi: 10.1002/jcp.27187. Epub 2018 Dec 24. J Cell Physiol. 2019. PMID: 30584668
-
Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-κB and Activating Nrf2/HO-1 Signaling Pathways.Front Pharmacol. 2021 Jan 29;11:591836. doi: 10.3389/fphar.2020.591836. eCollection 2020. Front Pharmacol. 2021. PMID: 33633565 Free PMC article.
-
Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway.Int Immunopharmacol. 2017 May;46:194-200. doi: 10.1016/j.intimp.2017.03.008. Int Immunopharmacol. 2017. PMID: 28315822
-
Kirenol ameliorates endotoxin-induced acute lung injury by inhibiting the ERK and JNK phosphorylation-mediated NFκB pathway in mice.Inflammopharmacology. 2025 Apr;33(4):2069-2081. doi: 10.1007/s10787-025-01693-2. Epub 2025 Mar 4. Inflammopharmacology. 2025. PMID: 40035943
-
Kirenol: A Potential Natural Lead Molecule for a New Drug Design, Development, and Therapy for Inflammation.Molecules. 2022 Jan 23;27(3):734. doi: 10.3390/molecules27030734. Molecules. 2022. PMID: 35163999 Free PMC article. Review.
Cited by
-
Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation.Front Pharmacol. 2021 Dec 23;12:790072. doi: 10.3389/fphar.2021.790072. eCollection 2021. Front Pharmacol. 2021. PMID: 35002723 Free PMC article.
-
Impact of time intervals on drug efficacy and phenotypic outcomes in acute respiratory distress syndrome in mice.Sci Rep. 2024 Sep 5;14(1):20768. doi: 10.1038/s41598-024-71659-x. Sci Rep. 2024. PMID: 39237657 Free PMC article.
-
Nrf2 Deficiency Exacerbated CLP-Induced Pulmonary Injury and Inflammation through Autophagy- and NF-κB/PPARγ-Mediated Macrophage Polarization.Cells. 2022 Dec 4;11(23):3927. doi: 10.3390/cells11233927. Cells. 2022. PMID: 36497185 Free PMC article.
-
Reactive Oxygen Species and Strategies for Antioxidant Intervention in Acute Respiratory Distress Syndrome.Antioxidants (Basel). 2023 Nov 18;12(11):2016. doi: 10.3390/antiox12112016. Antioxidants (Basel). 2023. PMID: 38001869 Free PMC article. Review.
-
Role of medicinal herbs and phytochemicals in post burn management.Inflammopharmacology. 2023 Aug;31(4):1695-1714. doi: 10.1007/s10787-023-01246-5. Epub 2023 May 19. Inflammopharmacology. 2023. PMID: 37204694 Review.
References
-
- Ni Y.L., Shen H.T., Su C.H., Chen W.Y., Huang-Liu R., Chen C.J., Chen S.P., Kuan Y.H. Nerolidol Suppresses the Inflammatory Response during Lipopolysaccharide-Induced Acute Lung Injury via the Modulation of Antioxidant Enzymes and the AMPK/Nrf2/HO-1 Pathway. Oxid. Med. Cell Longev. 2019;2019:9605980. doi: 10.1155/2019/9605980. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

