The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion
- PMID: 33572525
- PMCID: PMC7866784
- DOI: 10.3390/cancers13030535
The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion
Abstract
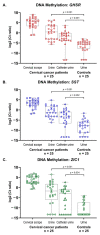
In urogenital cancers, urine as a liquid biopsy for non-invasive cancer detection holds great promise for future clinical application. Their anatomical position allows for the local shedding of tumor DNA, but recent data indicate that tumor DNA in urine might also result from transrenal excretion. This study aims to assess the origin of tumor-associated DNA in the urine of 5 bladder and 25 cervical cancer patients. Besides natural voided urine, paired urine samples were collected in which contact with the local tumor was circumvented to bypass local shedding. The latter concerned nephrostomy urine in bladder cancer patients, and catheter urine in cervical cancer patients. Methylation levels of GHSR, SST, and ZIC1 were determined using paired bladder tumor tissues and cervical scrapes as a reference. Urinary methylation levels were compared to natural voided urine of matched controls. To support methylation results, mutation analysis was performed in urine and tissue samples of bladder cancer patients. Increased methylation levels were not only found in natural voided urine from bladder and cervical cancer patients, but also in the corresponding nephrostomy and catheter urine. DNA mutations detected in bladder tumor tissues were also detectable in all paired natural voided urine as well as in a subset of nephrostomy urine. These results provide the first evidence that the suitability of urine as a liquid biopsy for urogenital cancers relies both on the local shedding of tumor cells and cell fragments, as well as the transrenal excretion of tumor DNA into the urine.
Keywords: biomarkers; liquid biopsy; methylation; molecular diagnostics; mutation; urine; urogenital neoplasms.
Conflict of interest statement
Renske D.M. Steenbergen has a minority share in Self-screen BV, a spin-off company of Amsterdam UMC, location VUmc.
Figures


Similar articles
-
Bladder cancer detection in urine using DNA methylation markers: a technical and prospective preclinical validation.Clin Epigenetics. 2022 Feb 5;14(1):19. doi: 10.1186/s13148-022-01240-8. Clin Epigenetics. 2022. PMID: 35123558 Free PMC article.
-
Oncoprotein DEK as a tissue and urinary biomarker for bladder cancer.BMC Cancer. 2011 Jun 10;11:234. doi: 10.1186/1471-2407-11-234. BMC Cancer. 2011. PMID: 21663673 Free PMC article.
-
Clinical sensitivity of p53 mutation detection in matched bladder tumor, bladder wash, and voided urine specimens.Cancer. 2001 Jun 1;91(11):2127-35. doi: 10.1002/1097-0142(20010601)91:11<2127::aid-cncr1241>3.0.co;2-r. Cancer. 2001. PMID: 11391594
-
Minimally Invasive and Emerging Diagnostic Approaches in Endometrial Cancer: Epigenetic Insights and the Promise of DNA Methylation.Diagnostics (Basel). 2024 Nov 15;14(22):2575. doi: 10.3390/diagnostics14222575. Diagnostics (Basel). 2024. PMID: 39594241 Free PMC article. Review.
-
DNA-Methylation-Based Detection of Urological Cancer in Urine: Overview of Biomarkers and Considerations on Biomarker Design, Source of DNA, and Detection Technologies.Int J Mol Sci. 2019 May 30;20(11):2657. doi: 10.3390/ijms20112657. Int J Mol Sci. 2019. PMID: 31151158 Free PMC article. Review.
Cited by
-
Liquid Biopsy, an Everchanging Balance between Clinical Utility and Emerging Technologies.Cancers (Basel). 2022 Sep 1;14(17):4277. doi: 10.3390/cancers14174277. Cancers (Basel). 2022. PMID: 36077819 Free PMC article.
-
Urine as a Source of Liquid Biopsy for Cancer.Cancers (Basel). 2021 May 28;13(11):2652. doi: 10.3390/cancers13112652. Cancers (Basel). 2021. PMID: 34071230 Free PMC article. Review.
-
A review of urinary HPV testing for cervical cancer management and HPV vaccine surveillance: rationale, strategies, and limitations.Eur J Clin Microbiol Infect Dis. 2024 Dec;43(12):2247-2258. doi: 10.1007/s10096-024-04963-z. Epub 2024 Oct 14. Eur J Clin Microbiol Infect Dis. 2024. PMID: 39400675 Review.
-
Research Progress of DNA Methylation Markers for Endometrial Carcinoma Diagnosis.J Cancer. 2025 Jan 1;16(3):812-820. doi: 10.7150/jca.104214. eCollection 2025. J Cancer. 2025. PMID: 39781343 Free PMC article. Review.
-
Unleashing the potential of urine DNA methylation detection: Advancements in biomarkers, clinical applications, and emerging technologies.Curr Urol. 2025 Sep;19(5):295-302. doi: 10.1097/CU9.0000000000000291. Epub 2025 Jul 19. Curr Urol. 2025. PMID: 40894279 Free PMC article.
References
-
- Bosschieter J., Lutz C., Segerink L.I., Vis A.N., Zwarthoff E.C., Van Moorselaar R.J.A., Van Rhijn B.W., Heymans M.W., Jansma E.P., Steenbergen R.D.M., et al. The diagnostic accuracy of methylation markers in urine for the detection of bladder cancer: A systematic review. Epigenomics. 2018;10:673–687. doi: 10.2217/epi-2017-0156. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

