Maternal Autogenous Inactivated Virus Vaccination Boosts Immunity to PRRSV in Piglets
- PMID: 33572562
- PMCID: PMC7912564
- DOI: 10.3390/vaccines9020106
Maternal Autogenous Inactivated Virus Vaccination Boosts Immunity to PRRSV in Piglets
Abstract
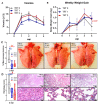
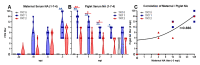
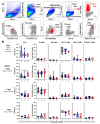
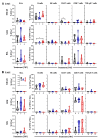
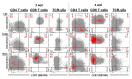
Maternal-derived immunity is a critical component for the survival and success of offspring in pigs to protect from circulating pathogens such as Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-2). The purpose of this study is to investigate the transfer of anti-PRRSV immunity to piglets from gilts that received modified-live virus (MLV) alone (treatment (TRT) 0), or in combination with one of two autogenous inactivated vaccines (AIVs, TRT 1+2). Piglets from these gilts were challenged with the autogenous PRRSV-2 strain at two weeks of age and their adaptive immune response (IR) was evaluated until 4 weeks post inoculation (wpi). The systemic humoral and cellular IR was analyzed in the pre-farrow gilts, and in piglets, pre-inoculation, and at 2 and 4 wpi. Both AIVs partially protected the piglets with reduced lung pathology and increased weight gain; TRT 1 also lowered piglet viremia, best explained by the AIV-induced production of neutralizing antibodies in gilts and their transfer to the piglets. In piglets, pre-inoculation, the main systemic IFN-γ producers were CD21α+ B cells. From 0 to 4 wpi, the role of these B cells declined and CD4 T cells became the primary systemic IFN-γ producers. In the lungs, CD8 T cells were the primary and CD4 T cells were the secondary IFN-γ producers, including a novel subset of porcine CD8α-CCR7- CD4 T cells, potentially terminally differentiated CD4 TEMRA cells. In summary, this study demonstrates that maternal AIV vaccination can improve protection of pre-weaning piglets against PRRSV-2; it shows the importance of transferring neutralizing antibodies to piglets, and it introduces two novel immune cell subsets in pigs-IFN-γ producing CD21α+ B cells and CD8α-CCR7- CD4 T cells.
Keywords: CD4 TEMRA cells; IFN-γ producing B cells; PRRSV; T cells; autogenous inactivated vaccine; cell-mediated immune response; humoral immune response; maternal vaccination; swine; transfer of immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
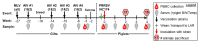

Similar articles
-
Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination.Vaccines (Basel). 2023 Mar 5;11(3):594. doi: 10.3390/vaccines11030594. Vaccines (Basel). 2023. PMID: 36992179 Free PMC article. Review.
-
Antibody response and maternal immunity upon boosting PRRSV-immune sows with experimental farm-specific and commercial PRRSV vaccines.Vet Microbiol. 2013 Dec 27;167(3-4):260-71. doi: 10.1016/j.vetmic.2013.08.017. Epub 2013 Aug 28. Vet Microbiol. 2013. PMID: 24041768
-
Cross-protection of a modified-live porcine reproductive and respiratory syndrome virus (PRRSV)-2 vaccine against a heterologous PRRSV-1 challenge in late-term pregnancy gilts.Vet Microbiol. 2018 Sep;223:119-125. doi: 10.1016/j.vetmic.2018.08.008. Epub 2018 Aug 6. Vet Microbiol. 2018. PMID: 30173737
-
Evaluation of PRRSv specific, maternally derived and induced immune response in Ingelvac PRRSFLEX EU vaccinated piglets in the presence of maternally transferred immunity.PLoS One. 2019 Oct 2;14(10):e0223060. doi: 10.1371/journal.pone.0223060. eCollection 2019. PLoS One. 2019. PMID: 31577832 Free PMC article.
-
Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):1-14. doi: 10.1016/j.vetimm.2015.07.003. Epub 2015 Jul 17. Vet Immunol Immunopathol. 2015. PMID: 26209116 Free PMC article. Review.
Cited by
-
Heterologous vaccine immunogenicity, efficacy, and immune correlates of protection of a modified-live virus porcine reproductive and respiratory syndrome virus vaccine.Front Microbiol. 2022 Sep 23;13:977796. doi: 10.3389/fmicb.2022.977796. eCollection 2022. Front Microbiol. 2022. PMID: 36212883 Free PMC article.
-
Gene expression of peripheral blood mononuclear cells and CD8+ T cells from gilts after PRRSV infection.Front Immunol. 2023 Jun 20;14:1159970. doi: 10.3389/fimmu.2023.1159970. eCollection 2023. Front Immunol. 2023. PMID: 37409113 Free PMC article.
-
Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination.Vaccines (Basel). 2023 Mar 5;11(3):594. doi: 10.3390/vaccines11030594. Vaccines (Basel). 2023. PMID: 36992179 Free PMC article. Review.
-
Correlation of Neutralizing Antibodies (NAbs) between Sows and Piglets and Evaluation of Protectability Associated with Maternally Derived NAbs in Pigs against Circulating Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) under Field Conditions.Vaccines (Basel). 2021 Apr 21;9(5):414. doi: 10.3390/vaccines9050414. Vaccines (Basel). 2021. PMID: 33919161 Free PMC article.
-
Challenges and Lessons Learned from a Field Trial on the Understanding of the Porcine Respiratory Disease Complex.Vaccines (Basel). 2025 Jul 9;13(7):740. doi: 10.3390/vaccines13070740. Vaccines (Basel). 2025. PMID: 40733717 Free PMC article.
References
-
- Merriam-Webster Inc . The Merriam-Webster Dictionary. Merriam-Webster, Incorporated; Springfield, MA, USA: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

