A Cellular Assay for the Identification and Characterization of Connexin Gap Junction Modulators
- PMID: 33572565
- PMCID: PMC7866863
- DOI: 10.3390/ijms22031417
A Cellular Assay for the Identification and Characterization of Connexin Gap Junction Modulators
Abstract
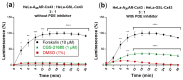
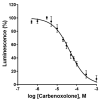
Connexin gap junctions (Cx GJs) enable the passage of small molecules and ions between cells and are therefore important for cell-to-cell communication. Their dysfunction is associated with diseases, and small molecules acting as modulators of GJs may therefore be useful as therapeutic drugs. To identify GJ modulators, suitable assays are needed that allow compound screening. In the present study, we established a novel assay utilizing HeLa cells recombinantly expressing Cx43. Donor cells additionally expressing the Gs protein-coupled adenosine A2A receptor, and biosensor cells expressing a cAMP-sensitive GloSensor luciferase were established. Adenosine A2A receptor activation in the donor cells using a selective agonist results in intracellular cAMP production. The negatively charged cAMP migrates via the Cx43 gap junctions to the biosensor cells and can there be measured by the cAMP-dependent luminescence signal. Cx43 GJ modulators can be expected to impact the transfer of cAMP from the donor to the biosensor cells, since cAMP transit is only possible via GJs. The new assay was validated by testing the standard GJ inhibitor carbenoxolon, which showed a concentration-dependent inhibition of the signal and an IC50 value that was consistent with previously reported values. The assay was demonstrated to be suitable for high-throughput screening.
Keywords: GloSensor luciferase; HeLa cells; compound library; connexin-43; gap junctions; screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

