Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches
- PMID: 33572583
- PMCID: PMC7911796
- DOI: 10.3390/md19020078
Marine Heterocyclic Compounds That Modulate Intracellular Calcium Signals: Chemistry and Synthesis Approaches
Abstract
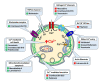
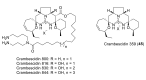
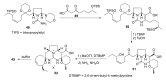
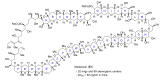
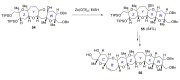
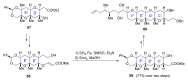
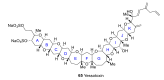
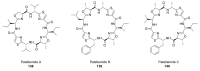
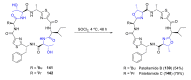
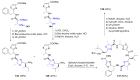
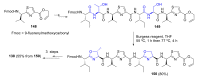
Intracellular Ca2+ plays a pivotal role in the control of a large series of cell functions in all types of cells, from neurotransmitter release and muscle contraction to gene expression, cell proliferation and cell death. Ca2+ is transported through specific channels and transporters in the plasma membrane and subcellular organelles such as the endoplasmic reticulum and mitochondria. Therefore, dysregulation of intracellular Ca2+ homeostasis may lead to cell dysfunction and disease. Accordingly, chemical compounds from natural origin and/or synthesis targeting directly or indirectly these channels and proteins may be of interest for the treatment of cell dysfunction and disease. In this review, we show an overview of a group of marine drugs that, from the structural point of view, contain one or various heterocyclic units in their core structure, and from the biological side, they have a direct influence on the transport of calcium in the cell. The marine compounds covered in this review are divided into three groups, which correspond with their direct biological activity, such as compounds with a direct influence in the calcium channel, compounds with a direct effect on the cytoskeleton and drugs with an effect on cancer cell proliferation. For each target, we describe its bioactive properties and synthetic approaches. The wide variety of chemical structures compiled in this review and their significant medical properties may attract the attention of many different researchers.
Keywords: calcium channel; heterocycles; marine drugs; medicinal properties; total synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

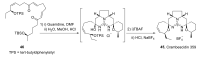
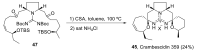



Similar articles
-
Synthesis of Medicinally Privileged Heterocycles through Dielectric Heating.Curr Med Chem. 2017;24(41):4596-4626. doi: 10.2174/0929867324666170223152137. Curr Med Chem. 2017. PMID: 28240166 Review.
-
Synthesis and effects on intracellular calcium of some 1,3-bis-(heteroaryl substituted)benzene derivatives.Farmaco. 2002 Jul;57(7):543-8. doi: 10.1016/s0014-827x(02)01246-6. Farmaco. 2002. PMID: 12164210
-
Pharmacological regulators of intracellular calcium release channels.Curr Pharm Des. 2007;13(24):2428-42. doi: 10.2174/138161207781368620. Curr Pharm Des. 2007. PMID: 17692011 Review.
-
Abnormal intracellular ca(2+)homeostasis and disease.Cell Calcium. 2000 Jul;28(1):1-21. doi: 10.1054/ceca.2000.0131. Cell Calcium. 2000. PMID: 10942700 Review.
-
Heterocyclic nucleosides: chemical synthesis and biological properties.Curr Med Chem. 2006;13(5):539-45. doi: 10.2174/092986706776055779. Curr Med Chem. 2006. PMID: 16515520 Review.
Cited by
-
Marine Pharmacology in 2019-2021: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action.Mar Drugs. 2024 Jun 30;22(7):309. doi: 10.3390/md22070309. Mar Drugs. 2024. PMID: 39057418 Free PMC article. Review.
-
Therapeutic Potential of Heterocyclic Compounds Targeting Mitochondrial Calcium Homeostasis and Signaling in Alzheimer's Disease and Parkinson's Disease.Antioxidants (Basel). 2023 Jun 15;12(6):1282. doi: 10.3390/antiox12061282. Antioxidants (Basel). 2023. PMID: 37372013 Free PMC article. Review.
-
Multi-tissue metabolomic profiling reveals the crucial metabolites and pathways associated with scallop growth.BMC Genomics. 2024 Nov 15;25(1):1091. doi: 10.1186/s12864-024-11016-4. BMC Genomics. 2024. PMID: 39548384 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

