Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination
- PMID: 33572639
- PMCID: PMC7911866
- DOI: 10.3390/vaccines9020107
Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination
Abstract
Background: Although glutamine is able to improve the immune response, its action in the upper airway immunity against the influenza virus vaccine remains unclear. Therefore, we aimed to evaluate the L-glutamine supplementation effect on the mucosal immune/inflammatory response of elderly subjects vaccinated against the influenza virus.
Methods: Saliva sampling from 83 physically active elderly volunteers were collected pre- and 30 days after influenza virus vaccination and supplementation with L-glutamine (Gln, n = 42) or placebo (PL, n = 41).
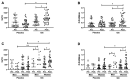
Results: Gln group showed higher salivary levels of interleukin (IL)-17, total secretory immunoglobulin A (SIgA), and specific-SIgA post-vaccination than values found pre-vaccination and in the PL group post-vaccination. Whereas higher salivary levels of IL-6 and IL-10 were observed post-vaccination in the Gln group, IL-37 levels were lower post-vaccination in both groups than the values pre-vaccination. Tumor necrosis factor (TNF)-α levels were unchanged. Positive correlations between IL-6 and IL-10 were found in all volunteer groups pre- and post-vaccination and also between IL-17 and IL-6 or IL-10 in the Gln group post-vaccination. A negative correlation between IL-37 and IL-10 was found pre- and post-vaccination in the PL group.
Conclusion: Gln supplementation was able to modulate salivary cytokine profile and increase SIgA levels, both total and specific to the influenza virus vaccine, in physically active elderly subjects.
Keywords: L-glutamine; antibodies; cytokines; immunoglobulin; influenza virus; vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- CDC. NCIRD . Vaccine Effectiveness: How Well Do the Flu Vaccines Work? U.S. Department of Health & Human Services; Washington, DC, USA: 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

