Cytoprotective Effects of Punicalagin on Hydrogen-Peroxide-Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells
- PMID: 33572785
- PMCID: PMC7911437
- DOI: 10.3390/antiox10020192
Cytoprotective Effects of Punicalagin on Hydrogen-Peroxide-Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells
Abstract
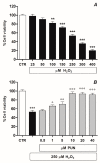
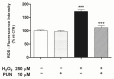
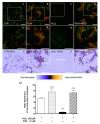
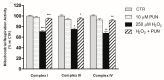
The retinal pigment epithelium (RPE) is a densely pigmented, monostratified epithelium that provides metabolic and functional support to the outer segments of photoreceptors. Endogenous or exogenous oxidative stimuli determine a switch from physiological to pathological conditions, characterized by an increase of intracellular levels of reactive oxygen species (ROS). Accumulating evidence has elucidated that punicalagin (PUN), the major ellagitannin in pomegranate, is a potent antioxidant in several cell types. The present study aimed to investigate the protective effect of PUN on mitochondrial dysfunction associated with hydrogen peroxide (H2O2)-induced oxidative stress. For this purpose, we used a human RPE cell line (ARPE-19) exposed to H2O2 for 24 h. The effects of PUN pre-treatment (24 h) were examined on cell viability, mitochondrial ROS levels, mitochondrial membrane potential, and respiratory chain complexes, then finally on caspase-3 enzymatic activity. The results showed that supplementation with PUN: (a) significantly increased cell viability; (b) kept the mitochondrial membrane potential (ΔΨm) at healthy levels and limited ROS production; (c) preserved the activity of respiratory complexes; (d) reduced caspase-3 activity. In conclusion, due to its activity in helping mitochondrial functions, reducing oxidative stress, and subsequent induction of cellular apoptosis, PUN might be considered a useful nutraceutical agent in the treatment of oxidation-associated disorders of RPE.
Keywords: ARPE–19 (human–RPE cell line); mitochondrion; oxidative stress; punicalagin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

