Incorporation of Calcium Sulfate Dihydrate into a Mesoporous Calcium Silicate/Poly-ε-Caprolactone Scaffold to Regulate the Release of Bone Morphogenetic Protein-2 and Accelerate Bone Regeneration
- PMID: 33572786
- PMCID: PMC7911692
- DOI: 10.3390/biomedicines9020128
Incorporation of Calcium Sulfate Dihydrate into a Mesoporous Calcium Silicate/Poly-ε-Caprolactone Scaffold to Regulate the Release of Bone Morphogenetic Protein-2 and Accelerate Bone Regeneration
Abstract
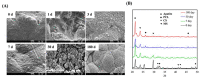
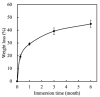
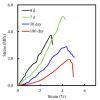
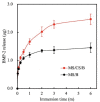
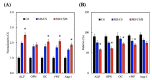
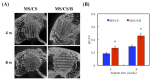
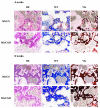
Tissue engineering and scaffolds play an important role in tissue regeneration by supporting cell adhesion, proliferation, and differentiation. The design of a scaffold is critical in determining its feasibility, and it is critical to note that each tissue is unique in terms of its morphology and composition. However, calcium-silicate-based scaffolds are undegradable, which severely limits their application in bone regeneration. In this study, we developed a biodegradable mesoporous calcium silicate (MS)/calcium sulfate (CS)/poly-ε-caprolactone (PCL) composite and fabricated a composite scaffold with 3D printing technologies. In addition, we were able to load bone morphogenetic protein-2 (BMP-2) into MS powder via a one-step immersion procedure. The results demonstrated that the MS/CS scaffold gradually degraded within 3 months. More importantly, the scaffold exhibited a gradual release of BMP-2 throughout the test period. The adhesion and proliferation of human dental pulp stem cells on the MS/CS/BMP-2 (MS/CS/B) scaffold were significantly greater than that on the MS/CS scaffold. It was also found that cells cultured on the MS/CS/B scaffold had significantly higher levels of alkaline phosphatase activity and angiogenic-related protein expression. The MS/CS/B scaffold promoted the growth of new blood vessels and bone regeneration within 4 weeks of implantation in rabbits with induced critical-sized femoral defects. Therefore, it is hypothesized that the 3D-printed MS/CS/B scaffold can act both as a conventional BMP-2 delivery system and as an ideal osteoinductive biomaterial for bone regeneration.
Keywords: 3D printing; bone morphogenetic protein-2; calcium silicate; calcium sulfate; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Wang W.C., Nune K.C., Tan L., Zhang N., Dong J., Yan J., Misra R.D.K., Yang K. Bone regeneration of hollow tubular magnesium-strontium scaffolds in critical-size segmental defects: Effect of surface coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;100:297–307. doi: 10.1016/j.msec.2019.02.067. - DOI - PubMed
-
- Gómez-Lizárraga K.K., Flores-Morales C., Del Prado-Audelo M.L., Álvarez-Pérez M.A., Piña-Barba M.C., Escobedo C. Polycaprolactone- and polycaprolactone/ceramic-based 3D-bioplotted porous scaffolds for bone regeneration: A comparative study. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;79:326–335. doi: 10.1016/j.msec.2017.05.003. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

