ALK2 Receptor Kinase Association with FKBP12.6 Is Structurally Conserved with the ALK2-FKBP12 Complex
- PMID: 33572801
- PMCID: PMC7911104
- DOI: 10.3390/biomedicines9020129
ALK2 Receptor Kinase Association with FKBP12.6 Is Structurally Conserved with the ALK2-FKBP12 Complex
Abstract
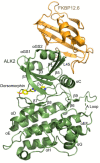
The immunophilin FKBP12 is a known inhibitor of type I BMP and TGF-β receptors that competes for binding with their substrate SMADs. FKBP12 and the close paralog FKBP12.6 additionally assemble with ryanodine receptors to control Ca2+ release. Binding of FKBP12.6 to BMP/TGF-β receptors has yet to be investigated, but appears plausible given its high sequence similarity to FKBP12. Here, we found that FKBP12.6 can assemble with BMP and TGF-β-family type I receptors, but not with type II receptors. Cellular immunoprecipitation confirmed similar binding of FKBP12 and FKBP12.6 to the BMP receptor ALK2 (ACVR1), a known target of mutations in the congenital syndrome fibrodysplasia ossificans progressiva (FOP), as well as the pediatric brain tumor diffuse intrinsic pontine glioma (DIPG). SEC-MALS analyses using purified proteins indicated a direct 1:1 interaction between FKBP12.6 and the receptor's cytoplasmic domains. The 2.17 Å structure of this ALK2-FKBP12.6 complex bound to the inhibitor dorsomorphin showed FKBP12.6 binding to the GS domain of ALK2 in a manner equivalent to the FKBP12 complex, with ALK2 residues Phe198 and Leu199 extending into the FK506-binding pocket of FKBP12.6. These findings suggest a level of redundancy in FKBP-family regulation of BMP and TGF-β signaling.
Keywords: ACVR1; ALK2; BMP; FKBP12; FKBP12.6; fibrodysplasia ossificans progressiva; kinase.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

