Orientation of Antigen Display on Self-Assembling Protein Nanoparticles Influences Immunogenicity
- PMID: 33572803
- PMCID: PMC7911071
- DOI: 10.3390/vaccines9020103
Orientation of Antigen Display on Self-Assembling Protein Nanoparticles Influences Immunogenicity
Abstract
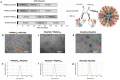
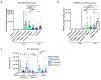
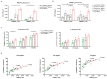
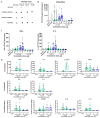
Self-assembling protein nanoparticles (SAPN) serve as a repetitive antigen delivery platform with high-density epitope display; however, antigen characteristics such as size and epitope presentation can influence the immunogenicity of the assembled particle and are aspects to consider for a rationally designed effective vaccine. Here, we characterize the folding and immunogenicity of heterogeneous antigen display by integrating (a) dual-stage antigen SAPN presenting the P. falciparum (Pf) merozoite surface protein 1 subunit, PfMSP119, and Pf cell-traversal protein for ookinetes and sporozoites, PfCelTOS, in addition to (b) a homogenous antigen SAPN displaying two copies of PfCelTOS. Mice and rabbits were utilized to evaluate antigen-specific humoral and cellular induction as well as functional antibodies via growth inhibition of the blood-stage parasite. We demonstrate that antigen orientation and folding influence the elicited immune response, and when appropriately designed, SAPN can serve as an adaptable platform for an effective multi-antigen display.
Keywords: PfCelTOS; PfMSP119; display; multi-stage; self-assembling protein nanoparticles; vaccine.
Conflict of interest statement
Peter Burkhard is the founder, co-owner, and CEO of Alpha-O Peptides AG, a company involved in nanoparticle vaccine design that holds intellectual property on the SAPN platform. The interpretations and opinions expressed herein belong to the authors and do not necessarily represent the official views of the U.S. Army, U.S. Navy, U.S. Department of Defense, or the U.S. government. Research was conducted in an AAALACi accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
Figures

References
-
- WHO . World Malaria Report 2020. World Health Organization; Geneva, Switzerland: 2020.
-
- Weiss D.J., Lucas T.C.D., Nguyen M., Nandi A.K., Bisanzio D., Battle K.E., Cameron E., Twohig K.A., Pfeffer D.A., Rozier J.A., et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000–2017: A spatial and temporal modelling study. Lancet. 2019;394:322–331. doi: 10.1016/S0140-6736(19)31097-9. - DOI - PMC - PubMed
-
- Zinszer K., Charland K., Vahey S., Jahagirdar D., Rek J.C., Arinaitwe E., Nankabirwa J., Morrison K., Sadoine M.L., Tutt-Guerette M.A., et al. The Impact of Multiple Rounds of Indoor Residual Spraying on Malaria Incidence and Hemoglobin Levels in a High-Transmission Setting. J. Infect. Dis. 2020;221:304–312. doi: 10.1093/infdis/jiz453. - DOI - PMC - PubMed
-
- Kenangalem E., Poespoprodjo J.R., Douglas N.M., Burdam F.H., Gdeumana K., Chalfein F., Prayoga, Thio F., Devine A., Marfurt J., et al. Malaria morbidity and mortality following introduction of a universal policy of artemisinin-based treatment for malaria in Papua, Indonesia: A longitudinal surveillance study. PLoS Med. 2019;16:e1002815. doi: 10.1371/journal.pmed.1002815. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

