Krill Oil Supplementation Reduces Exacerbated Hepatic Steatosis Induced by Thermoneutral Housing in Mice with Diet-Induced Obesity
- PMID: 33572810
- PMCID: PMC7912192
- DOI: 10.3390/nu13020437
Krill Oil Supplementation Reduces Exacerbated Hepatic Steatosis Induced by Thermoneutral Housing in Mice with Diet-Induced Obesity
Abstract
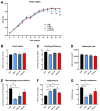
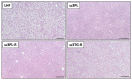
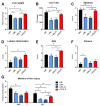
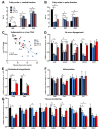
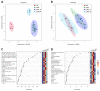
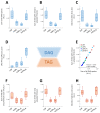
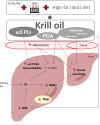
Preclinical evidence suggests that n-3 fatty acids EPA and DHA (Omega-3) supplemented as phospholipids (PLs) may be more effective than triacylglycerols (TAGs) in reducing hepatic steatosis. To further test the ability of Omega-3 PLs to alleviate liver steatosis, we used a model of exacerbated non-alcoholic fatty liver disease based on high-fat feeding at thermoneutral temperature. Male C57BL/6N mice were fed for 24 weeks a lard-based diet given either alone (LHF) or supplemented with Omega-3 (30 mg/g diet) as PLs (krill oil; ω3PL) or TAGs (Epax 3000TG concentrate; ω3TG), which had a similar total content of EPA and DHA and their ratio. Substantial levels of TAG accumulation (~250 mg/g) but relatively low inflammation/fibrosis levels were achieved in the livers of control LHF mice. Liver steatosis was reduced by >40% in the ω3PL but not ω3TG group, and plasma ALT levels were markedly reduced (by 68%) in ω3PL mice as well. Krill oil administration also improved hepatic insulin sensitivity, and its effects were associated with high plasma adiponectin levels (150% of LHF mice) along with superior bioavailability of EPA, increased content of alkaloids stachydrine and trigonelline, suppression of lipogenic gene expression, and decreased diacylglycerol levels in the liver. This study reveals that in addition to Omega-3 PLs, other constituents of krill oil, such as alkaloids, may contribute to its strong antisteatotic effects in the liver.
Keywords: C57BL/6N mice; NAFLD; high-fat diet; krill oil; obesity; omega-3; phospholipids; thermoneutral temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Omega-3 Phospholipids from Krill Oil Enhance Intestinal Fatty Acid Oxidation More Effectively than Omega-3 Triacylglycerols in High-Fat Diet-Fed Obese Mice.Nutrients. 2020 Jul 9;12(7):2037. doi: 10.3390/nu12072037. Nutrients. 2020. PMID: 32660007 Free PMC article.
-
Increased plasma levels of palmitoleic acid may contribute to beneficial effects of Krill oil on glucose homeostasis in dietary obese mice.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Aug;1865(8):158732. doi: 10.1016/j.bbalip.2020.158732. Epub 2020 May 1. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32371092
-
Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice.Biochim Biophys Acta. 2014 Feb;1841(2):267-78. doi: 10.1016/j.bbalip.2013.11.010. Biochim Biophys Acta. 2014. PMID: 24295779
-
Influence of Lipid Class Used for Omega-3 Fatty Acid Supplementation on Liver Fat Accumulation in MASLD.Physiol Res. 2024 Aug 31;73(Suppl 1):S295-S320. doi: 10.33549/physiolres.935396. Epub 2024 Jul 17. Physiol Res. 2024. PMID: 39016154 Free PMC article. Review.
-
Impact of the Co-Administration of N-3 Fatty Acids and Olive Oil Components in Preclinical Nonalcoholic Fatty Liver Disease Models: A Mechanistic View.Nutrients. 2020 Feb 15;12(2):499. doi: 10.3390/nu12020499. Nutrients. 2020. PMID: 32075238 Free PMC article. Review.
Cited by
-
Preventive and Therapeutic Effects of Krill Oil on Obesity and Obesity-Induced Metabolic Syndromes in High-Fat Diet-Fed Mice.Mar Drugs. 2022 Jul 27;20(8):483. doi: 10.3390/md20080483. Mar Drugs. 2022. PMID: 36005486 Free PMC article.
-
Energy Metabolism and Diet.Nutrients. 2021 Jun 1;13(6):1907. doi: 10.3390/nu13061907. Nutrients. 2021. PMID: 34206013 Free PMC article.
-
Obesity alters adipose tissue response to fasting and refeeding in women: A study on lipolytic and endocrine dynamics and acute insulin resistance.Heliyon. 2024 Sep 14;10(18):e37875. doi: 10.1016/j.heliyon.2024.e37875. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39328508 Free PMC article.
-
Optimization of Mobile Phase Modifiers for Fast LC-MS-Based Untargeted Metabolomics and Lipidomics.Int J Mol Sci. 2023 Jan 19;24(3):1987. doi: 10.3390/ijms24031987. Int J Mol Sci. 2023. PMID: 36768308 Free PMC article.
-
Krill Oil Inhibits Cholesterol Synthesis and Stimulated Cholesterol Excretion in Hypercholesterolemic Rats.Mar Drugs. 2022 Sep 27;20(10):609. doi: 10.3390/md20100609. Mar Drugs. 2022. PMID: 36286433 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

