Model Surfaces for Paper Fibers Prepared from Carboxymethyl Cellulose and Polycations
- PMID: 33573003
- PMCID: PMC7866410
- DOI: 10.3390/polym13030435
Model Surfaces for Paper Fibers Prepared from Carboxymethyl Cellulose and Polycations
Abstract
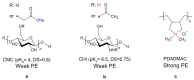
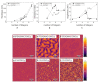
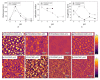
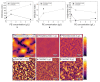
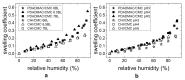
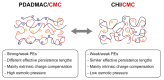
For tailored functionalization of cellulose based papers, the interaction between paper fibers and functional additives must be understood. Planar cellulose surfaces represent a suitable model system for studying the binding of additives. In this work, polyelectrolyte multilayers (PEMs) are prepared by alternating dip-coating of the negatively charged cellulose derivate carboxymethyl cellulose and a polycation, either polydiallyldimethylammonium chloride (PDADMAC) or chitosan (CHI). The parameters varied during PEM formation are the concentrations (0.1-5 g/L) and pH (pH = 2-6) of the dipping solutions. Both PEM systems grow exponentially, revealing a high mobility of the polyelectrolytes (PEs). The pH-tunable charge density leads to PEMs with different surface topographies. Quartz crystal microbalance experiments with dissipation monitoring (QCM-D) reveal the pronounced viscoelastic properties of the PEMs. Ellipsometry and atomic force microscopy (AFM) measurements show that the strong and highly charged polycation PDADMAC leads to the formation of smooth PEMs. The weak polycation CHI forms cellulose model surfaces with higher film thicknesses and a tunable roughness. Both PEM systems exhibit a high water uptake when exposed to a humid environment, with the PDADMAC/carboxymethyl cellulose (CMC) PEMs resulting in a water uptake up to 60% and CHI/CMC up to 20%. The resulting PEMs are water-stable, but water swellable model surfaces with a controllable roughness and topography.
Keywords: carboxymethyl cellulose; cellulose model surface; dip-coating; polyelectrolyte multilayers.
Conflict of interest statement
The authors have no conflict to declare.
Figures


Similar articles
-
Influence of Surface Charge Density and Morphology on the Formation of Polyelectrolyte Multilayers on Smooth Charged Cellulose Surfaces.Langmuir. 2017 Jan 31;33(4):968-979. doi: 10.1021/acs.langmuir.6b04217. Epub 2017 Jan 13. Langmuir. 2017. PMID: 28045539
-
The Princess and the Pea Effect: Influence of the first layer on polyelectrolyte multilayer assembly and properties.J Colloid Interface Sci. 2017 Sep 15;502:165-171. doi: 10.1016/j.jcis.2017.04.091. Epub 2017 Apr 29. J Colloid Interface Sci. 2017. PMID: 28482189
-
Quartz crystal microbalance with dissipation monitoring and surface plasmon resonance studies of carboxymethyl cellulose adsorption onto regenerated cellulose surfaces.Langmuir. 2011 Jul 19;27(14):8718-28. doi: 10.1021/la200628a. Epub 2011 Jun 23. Langmuir. 2011. PMID: 21699205
-
Influence of Molecular Weight on the Performance of Polyelectrolyte Multilayer Nanofiltration Membranes.ACS Appl Polym Mater. 2022 May 13;4(5):2962-2971. doi: 10.1021/acsapm.1c00826. Epub 2021 Sep 23. ACS Appl Polym Mater. 2022. PMID: 35601465 Free PMC article. Review.
-
A Perspective on the Glass Transition and the Dynamics of Polyelectrolyte Multilayers and Complexes.Langmuir. 2023 Oct 24;39(42):14823-14839. doi: 10.1021/acs.langmuir.3c00974. Epub 2023 Oct 11. Langmuir. 2023. PMID: 37819874 Free PMC article. Review.
Cited by
-
Transparent Biocompatible Polyelectrolyte Multilayer Coatings on Apples: Formation and Properties.ACS Food Sci Technol. 2025 Mar 1;5(3):1156-1165. doi: 10.1021/acsfoodscitech.4c01027. eCollection 2025 Mar 21. ACS Food Sci Technol. 2025. PMID: 40143962 Free PMC article.
References
-
- Dufresne A. Monomers, Polymers and Composites from Renewable Resources. Elsevier; Amsterdam, The Netherlands: 2008. Cellulose-Based Composites and Nanocomposites; pp. 401–418. - DOI
-
- Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008;57:397–430. doi: 10.1002/pi.2378. - DOI
-
- Dunlop-Jones N. Paper Chemistry. Springer; Dordrecht, The Netherlands: 1991. Wet-strength chemistry; pp. 76–96. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

