Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells
- PMID: 33573005
- PMCID: PMC7919370
- DOI: 10.3390/nano11020343
Real-Time Tracking of Highly Luminescent Mesoporous Silica Particles Modified with Europium β-Diketone Chelates in Living Cells
Abstract
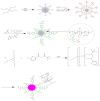
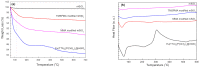
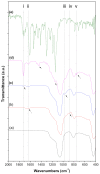
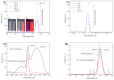
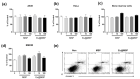
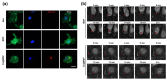
Highly luminescent europium complexes modified mesoporous silica particles (MSP) were synthesized as an imaging probes for both in-vitro diagnostic and in-vivo cellular tracking agents. Europium β-diketone chelates (4,4,4-trifluoro-l-(2-thienyl)-l,3-butanedione) trioctylphosphine europium (III) (Eu(TTA)3(P(Oct)3)3) were incorporated inside the nanocavities that existed in hierarchical MSP (Eu@MSP). The MSP and Eu@MSP on mouse bone marrow-derived macrophages (BMDMs) did not show any toxic effect. The MSP and Eu@MSP in the BMDMs were found at cytoplasm without any degradation and immunogenicity. However, both pro- and anti-inflammatory cytokines of macrophages were significantly increased when lipopolysaccharide and a high concentration (100 μg/mL) of MSP and Eu@MSP were treated simultaneously.
Keywords: beta-diketone; europium complex; inflammation; live cell image; mesoporous silica.
Conflict of interest statement
The authors declare that they have no known conflict of interest associated with this publication and there has been no competing financial interest for this work that could have influenced its outcome.
Figures

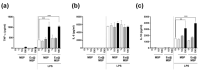
References
-
- Cao Z., Li B., Sun L., Li L., Xu Z.P., Gu Z. 2D Layered Double Hydroxide Nanoparticles: Recent Progress toward Preclinical/Clinical Nanomedicine. Small Methods. 2020;4:1900343. doi: 10.1002/smtd.201900343. - DOI
-
- Arsalani S., Oliveira J., Guidelli E.J., Araujo J.F.D.F., Wiekhorst F., Baffa O. Synthesis of radioluminescent iron oxide nanoparticles functionalized by anthracene for biomedical applications. Coll. Surf. A Physicochem. Eng. Asp. 2020;602:125105. doi: 10.1016/j.colsurfa.2020.125105. - DOI
-
- Chen L., Xu J., Wang Y., Huang R. Ultra-small MoS2 nanodots-incorporated mesoporous silica nanospheres for pH-sensitive drug delivery and CT imaging. J. Mater. Sci. Technol. 2020 doi: 10.1016/j.jmst.2020.03.019. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

