Monokaryotic Pleurotus sapidus Strains with Intraspecific Variability of an Alkene Cleaving DyP-Type Peroxidase Activity as a Result of Gene Mutation and Differential Gene Expression
- PMID: 33573012
- PMCID: PMC7866418
- DOI: 10.3390/ijms22031363
Monokaryotic Pleurotus sapidus Strains with Intraspecific Variability of an Alkene Cleaving DyP-Type Peroxidase Activity as a Result of Gene Mutation and Differential Gene Expression
Abstract
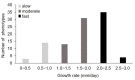
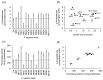
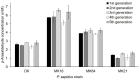
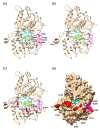
The basidiomycete Pleurotus sapidus produced a dye-decolorizing peroxidase (PsaPOX) with alkene cleavage activity, implying potential as a biocatalyst for the fragrance and flavor industry. To increase the activity, a daughter-generation of 101 basidiospore-derived monokaryons (MK) was used. After a pre-selection according to the growth rate, the activity analysis revealed a stable intraspecific variability of the strains regarding peroxidase and alkene cleavage activity of PsaPOX. Ten monokaryons reached activities up to 2.6-fold higher than the dikaryon, with MK16 showing the highest activity. Analysis of the PsaPOX gene identified three different enzyme variants. These were co-responsible for the observed differences in activities between strains as verified by heterologous expression in Komagataella phaffii. The mutation S371H in enzyme variant PsaPOX_high caused an activity increase alongside a higher protein stability, while the eleven mutations in variant PsaPOX_low resulted in an activity decrease, which was partially based on a shift of the pH optimum from 3.5 to 3.0. Transcriptional analysis revealed the increased expression of PsaPOX in MK16 as reason for the higher PsaPOX activity in comparison to other strains producing the same PsaPOX variant. Thus, different expression profiles, as well as enzyme variants, were identified as crucial factors for the intraspecific variability of the PsaPOX activity in the monokaryons.
Keywords: Pleurotus sapidus; alkene cleavage; basidiomycota; biocatalysis; dikaryon; dye-decolorizing peroxidase (DyP); gene expression; gene mutation; intraspecific variability; monokaryon.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Fahlbusch K.-G., Hammerschmidt F.-J., Panten J., Pickenhagen W., Schatkowski D., Bauer K., Garbe D., Surburg H. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2003. Flavors and Fragrances; pp. 73–140.
-
- Rajagopalan A., Lara M., Kroutil W. Oxidative Alkene Cleavage by Chemical and Enzymatic Methods. Adv. Synth. Catal. 2013;355:3321–3335. doi: 10.1002/adsc.201300882. - DOI
-
- Spannring P., Bruijnincx P.C.A., Weckhuysen B.M., Klein Gebbink R.J.M. Transition metal-catalyzed oxidative double bond cleavage of simple and bio-derived alkenes and unsaturated fatty acids. Catal. Sci. Technol. 2014;4:2182–2209. doi: 10.1039/c3cy01095c. - DOI
-
- Bel-Rhlid R., Berger R.G., Blank I. Bio-mediated generation of food flavors—Towards sustainable flavor production inspired by nature. Trends Food Sci. Technol. 2018;78:134–143. doi: 10.1016/j.tifs.2018.06.004. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

