Ustilaginaceae Biocatalyst for Co-Metabolism of CO2-Derived Substrates toward Carbon-Neutral Itaconate Production
- PMID: 33573033
- PMCID: PMC7911105
- DOI: 10.3390/jof7020098
Ustilaginaceae Biocatalyst for Co-Metabolism of CO2-Derived Substrates toward Carbon-Neutral Itaconate Production
Abstract
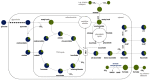
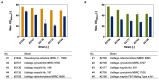
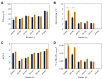
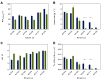
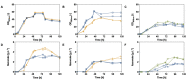
The family Ustilaginaceae (belonging to the smut fungi) are known for their plant pathogenicity. Despite the fact that these plant diseases cause agricultural yield reduction, smut fungi attracted special attention in the field of industrial biotechnology. Ustilaginaceae show a versatile product spectrum such as organic acids (e.g., itaconate, malate, succinate), polyols (e.g., erythritol, mannitol), and extracellular glycolipids, which are considered value-added chemicals with potential applications in the pharmaceutical, food, and chemical industries. This study focused on itaconate as a platform chemical for the production of resins, plastics, adhesives, and biofuels. During this work, 72 different Ustilaginaceae strains from 36 species were investigated for their ability to (co-) consume the CO2-derived substrates acetate and formate, potentially contributing toward a carbon-neutral itaconate production. The fungal growth and product spectrum with special interest in itaconate was characterized. Ustilago maydis MB215 and Ustilago rabenhorstiana NBRC 8995 were identified as promising candidates for acetate metabolization whereas Ustilago cynodontis NBRC 7530 was identified as a potential production host using formate as a co-substrate enhancing the itaconate production. Selected strains with the best itaconate production were characterized in more detail in controlled-batch bioreactor experiments confirming the co-substrate utilization. Thus, a proof-of-principle study was performed resulting in the identification and characterization of three promising Ustilaginaceae biocatalyst candidates for carbon-neutral itaconate production contributing to the biotechnological relevance of Ustilaginaceae.
Keywords: CO2; Ustilaginaceae; Ustilago maydis; biodiversity; chassis; itaconate; organic acid; smut fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

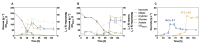

References
-
- Hosseinpour Tehrani H., Becker J., Bator I., Saur K., Meyer S., Rodrigues Lóia A.C., Blank L.M., Wierckx N. Integrated strain- and process design enable production of 220 g L−1 itaconic acid with Ustilago maydis. Biotechnol. Biofuels. 2019;12:263. doi: 10.1186/s13068-019-1605-6. - DOI - PMC - PubMed
-
- Robert T., Friebel S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016;18:2922–2934. doi: 10.1039/C6GC00605A. - DOI
-
- Steiger M.G., Wierckx N., Blank L.M., Mattanovich D., Sauer M. Industrial Biotechnology: Products and Processes. Wiley; Hoboken, NJ, USA: 2016. Itaconic acid an emerging building block. Chapter 15. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

