New 1,2,3-Triazole-Containing Hybrids as Antitumor Candidates: Design, Click Reaction Synthesis, DFT Calculations, and Molecular Docking Study
- PMID: 33573040
- PMCID: PMC7866392
- DOI: 10.3390/molecules26030708
New 1,2,3-Triazole-Containing Hybrids as Antitumor Candidates: Design, Click Reaction Synthesis, DFT Calculations, and Molecular Docking Study
Abstract
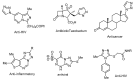
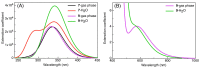
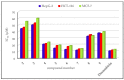
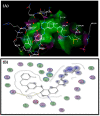
In an effort to improve and achieve biologically active anticancer agents, a novel series of 1,2,3-triazole-containing hybrids were designed and efficiently synthesized via the Cu-catalyzed azide-alkyne cycloaddition (CuAAC) reaction of substituted-arylazides with alkyne-functionalized pyrazole-[1,2,4]-triazole hybrids. The structure geometry of these new clicked 1,2,3-triazoles was explored by density functional theory (DFT) using the B3LYP/6-311++G(d,p) level; also, the potential activity of the compounds for light absorption was simulated by time-dependent DFT calculations (TD-DFT). The antitumor impacts of the newly synthesized compounds were in vitro estimated to be towards the human liver cancer cell line (HepG-2), the human colon cancer cell line (HCT-116), and human breast adenocarcinoma (MCF-7). Among the tested compounds, conjugate 7 was the most potent cytotoxic candidate towards HepG-2, HCT-116, and MCF-7, with IC50 = 12.22, 14.16, and 14.64 µM, respectively, in comparison to that exhibited by the standard drug doxorubicin (IC50 = 11.21, 12.46, and 13.45 µM). Finally, a molecular docking study was conducted within the epidermal growth factor receptor (EGFR) active site to suggest possible binding modes. Hence, it could conceivably be hypothesized that analogies 7, 6, and 5 could be considered as decent lead candidate compounds for anticancer agents.
Keywords: 1,2,3-triazole; DFT calculation; antitumor activity; click chemistry; cycloaddition reaction; molecular docking; pyrazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Boraei A.T., Gomaa M.S., El Sayed H., Duerkop A. Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur. J. Med. Chem. 2017;125:360–371. doi: 10.1016/j.ejmech.2016.09.046. - DOI - PubMed
-
- Aouad M.R., Mayaba M.M., Naqvi A., Bardaweel S.K., Al-blewi F.F., Messali M., Rezki N. Design, synthesis, in silico and in vitro antimicrobial screenings of novel 1,2,4-triazoles carrying 1,2,3-triazole scaffold with lipophilic side chain tether. Chem. Cent. J. 2017;11:117–123. doi: 10.1186/s13065-017-0347-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

