Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium
- PMID: 33573079
- PMCID: PMC7911484
- DOI: 10.3390/diagnostics11020203
Thromboinflammation Model-on-A-Chip by Whole Blood Microfluidics on Fixed Human Endothelium
Abstract
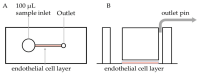
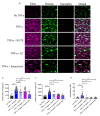
Microfluidic devices have an established role in the study of platelets and coagulation factors in thrombosis, with potential diagnostic applications. However, few microfluidic devices have assessed the contribution of neutrophils to thrombus formation, despite increasing knowledge of neutrophils' importance in cardiovascular thrombosis. We describe a thromboinflammation model which uses straight channels, lined with fixed human umbilical vein endothelial cells, after treatment with tumour necrosis factor-alpha. Re-calcified whole blood is perfused over the endothelium at venous and arterial shear rate. Neutrophil adhesion, platelet and fibrin thrombus formation, is measured over time by the addition of fluorescent antibodies to a whole blood sample. Fixed endothelium retains surface expression of adhesion molecules ICAM-1 and E-Selectin. Neutrophils adhere preferentially to platelet thrombi on the endothelium. Inhibitors of neutrophil adhesion and anti-inflammatory agents, such as isoquercetin, decrease neutrophil adhesion. Our model offers the advantage of the use of (1) fixed endothelium, (2) whole blood, instead of isolated neutrophils, and (3) a small amount of blood (1 mL). The characteristics of this thromboinflammation model provide the potential for further development for drug screening and point-of-care applications.
Keywords: endothelium; microfluidics; neutrophil; platelet; thromboinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium.Biomed Microdevices. 2016 Aug;18(4):73. doi: 10.1007/s10544-016-0095-6. Biomed Microdevices. 2016. PMID: 27464497 Free PMC article.
-
Microfluidic post method for 3-dimensional modeling of platelet-leukocyte interactions.Analyst. 2022 Mar 14;147(6):1222-1235. doi: 10.1039/d2an00270a. Analyst. 2022. PMID: 35212697
-
Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin.Circulation. 1998 Sep 1;98(9):873-82. doi: 10.1161/01.cir.98.9.873. Circulation. 1998. PMID: 9738642
-
Platelet-Neutrophil Interaction and Thromboinflammation in Diabetes: Considerations for Novel Therapeutic Approaches.J Am Heart Assoc. 2022 Oct 18;11(20):e027071. doi: 10.1161/JAHA.122.027071. Epub 2022 Oct 17. J Am Heart Assoc. 2022. PMID: 36250653 Free PMC article. Review.
-
P2X1: a unique platelet receptor with a key role in thromboinflammation.Platelets. 2021 Oct 3;32(7):902-908. doi: 10.1080/09537104.2021.1902972. Epub 2021 Mar 24. Platelets. 2021. PMID: 33760688 Review.
Cited by
-
Vascular dysfunction in hemorrhagic viral fevers: opportunities for organotypic modeling.Biofabrication. 2024 Jun 5;16(3):032008. doi: 10.1088/1758-5090/ad4c0b. Biofabrication. 2024. PMID: 38749416 Free PMC article. Review.
-
Platelet Mechanobiology Inspired Microdevices: From Hematological Function Tests to Disease and Drug Screening.Front Pharmacol. 2022 Jan 20;12:779753. doi: 10.3389/fphar.2021.779753. eCollection 2021. Front Pharmacol. 2022. PMID: 35126120 Free PMC article. Review.
-
Single molecule studies of dynamic platelet interactions with endothelial cells.Front Bioeng Biotechnol. 2024 Apr 3;12:1372807. doi: 10.3389/fbioe.2024.1372807. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38638321 Free PMC article.
-
Endothelial cell activation enhances thromboinflammation in vaccine-induced immune thrombotic thrombocytopenia.Blood Adv. 2025 Jun 24;9(12):2891-2906. doi: 10.1182/bloodadvances.2024014165. Blood Adv. 2025. PMID: 40085945 Free PMC article.
-
Emerging Microfluidic Approaches for Platelet Mechanobiology and Interplay With Circulatory Systems.Front Cardiovasc Med. 2021 Nov 25;8:766513. doi: 10.3389/fcvm.2021.766513. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34901226 Free PMC article. Review.
References
-
- Ghasemzadeh M., Kaplan Z.S., Alwis I., Schoenwaelder S.M., Ashworth K.J., Westein E., Hosseini E., Salem H.H., Slattery R., McColl S.R., et al. The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood. 2013;121:4555–4566. doi: 10.1182/blood-2012-09-459636. - DOI - PMC - PubMed
-
- Vital S.A., Becker F., Holloway P.M., Russell J., Perretti M., Granger N., Gavins F. Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation. 2016;133:2169–2179. doi: 10.1161/CIRCULATIONAHA.115.020633. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

