Role of CgTpo4 in Polyamine and Antimicrobial Peptide Resistance: Determining Virulence in Candida glabrata
- PMID: 33573089
- PMCID: PMC7866538
- DOI: 10.3390/ijms22031376
Role of CgTpo4 in Polyamine and Antimicrobial Peptide Resistance: Determining Virulence in Candida glabrata
Abstract
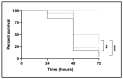
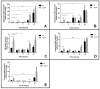
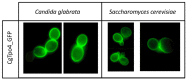
Candida glabrata is an emerging fungal pathogen whose success depends on its ability to resist antifungal drugs but also to thrive against host defenses. In this study, the predicted multidrug transporter CgTpo4 (encoded by ORF CAGL0L10912g) is described as a new determinant of virulence in C. glabrata, using the infection model Galleria mellonella. The CgTPO4 gene was found to be required for the C. glabrata ability to kill G. mellonella. The transporter encoded by this gene is also necessary for antimicrobial peptide (AMP) resistance, specifically against histatin-5. Interestingly, G. mellonella's AMP expression was found to be strongly activated in response to C. glabrata infection, suggesting AMPs are a key antifungal defense. CgTpo4 was also found to be a plasma membrane exporter of polyamines, especially spermidine, suggesting that CgTpo4 is able to export polyamines and AMPs, thus conferring resistance to both stress agents. Altogether, this study presents the polyamine exporter CgTpo4 as a determinant of C. glabrata virulence, which acts by protecting the yeast cells from the overexpression of AMPs, deployed as a host defense mechanism.
Keywords: AMP resistance; Candida glabrata; CgTpo4; Galleria mellonella; polyamine resistance; virulence.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Arendrup M.C., Garcia-Effron G., Lass-Florl C., Lopez A.G., Rodriguez-Tudela J.-L., Cuenca-Estrella M., Perlin D.S. Echinocandin Susceptibility Testing of Candida Species: Comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, Disk Diffusion, and Agar Dilution Methods with RPMI and IsoSensitest Media. Antimicrob. Agents Chemother. 2010;54:426–439. doi: 10.1128/AAC.01256-09. - DOI - PMC - PubMed
-
- Tscherner M., Schwarzmüller T., Kuchler K. Pathogenesis and Antifungal Drug Resistance of the Human Fungal Pathogen Candida glabrata. Pharmaceuticals. 2011;4:169–186. doi: 10.3390/ph4010169. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

