A Review on Ionic Liquid Gas Separation Membranes
- PMID: 33573138
- PMCID: PMC7911519
- DOI: 10.3390/membranes11020097
A Review on Ionic Liquid Gas Separation Membranes
Abstract
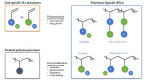
Ionic liquids have attracted the attention of the industry and research community as versatile solvents with unique properties, such as ionic conductivity, low volatility, high solubility of gases and vapors, thermal stability, and the possibility to combine anions and cations to yield an almost endless list of different structures. These features open perspectives for numerous applications, such as the reaction medium for chemical synthesis, electrolytes for batteries, solvent for gas sorption processes, and also membranes for gas separation. In the search for better-performing membrane materials and membranes for gas and vapor separation, ionic liquids have been investigated extensively in the last decade and a half. This review gives a complete overview of the main developments in the field of ionic liquid membranes since their first introduction. It covers all different materials, membrane types, their preparation, pure and mixed gas transport properties, and examples of potential gas separation applications. Special systems will also be discussed, including facilitated transport membranes and mixed matrix membranes. The main strengths and weaknesses of the different membrane types will be discussed, subdividing them into supported ionic liquid membranes (SILMs), poly(ionic liquids) or polymerized ionic liquids (PILs), polymer/ionic liquid blends (physically or chemically cross-linked 'ion-gels'), and PIL/IL blends. Since membrane processes are advancing as an energy-efficient alternative to traditional separation processes, having shown promising results for complex new separation challenges like carbon capture as well, they may be the key to developing a more sustainable future society. In this light, this review presents the state-of-the-art of ionic liquid membranes, to analyze their potential in the gas separation processes of the future.
Keywords: gas separation; ion gel membrane; ionic liquid; ionic liquid blends; polymerized ionic liquids; transport properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Wilkes J.S., Leviski J.A., Landers J.S., Hussey C.L., Vaughn R.L., Floreani D.J., Stech D.J. A new class of room temperature molten salts for battery applications; Proceedings of the AFSC/NAVHAT Science and Engineering Symposium; Colorado Springs, CO, USA. 27–29 October 1981; pp. 1–33.
-
- Wilkes J.S. Molten Salts and Ionic Liquids—Are They not the Same Thing? ECS Trans. 2007;3:3–7. doi: 10.1149/1.2798641. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

