Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients
- PMID: 33573162
- PMCID: PMC7911715
- DOI: 10.3390/life11020105
Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients
Abstract
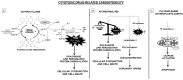
Reactive oxygen species (ROS) are molecules involved in signal transduction pathways with both beneficial and detrimental effects on human cells. ROS are generated by many cellular processes including mitochondrial respiration, metabolism and enzymatic activities. In physiological conditions, ROS levels are well-balanced by antioxidative detoxification systems. In contrast, in pathological conditions such as cardiovascular, neurological and cancer diseases, ROS production exceeds the antioxidative detoxification capacity of cells, leading to cellular damages and death. In this review, we will first describe the biology and mechanisms of ROS mediated oxidative stress in cardiovascular disease. Second, we will review the role of oxidative stress mediated by oncological treatments in inducing cardiovascular disease. Lastly, we will discuss the strategies that potentially counteract the oxidative stress in order to fight the onset and progression of cardiovascular disease, including that induced by oncological treatments.
Keywords: ROS; antioxidative treatment; cardiotoxicity; cardiovascular disease; endothelial dysfunction; oncological treatments; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

