The Effect of Neddylation Blockade on Slug-Dependent Cancer Cell Migration Is Regulated by p53 Mutation Status
- PMID: 33573293
- PMCID: PMC7866814
- DOI: 10.3390/cancers13030531
The Effect of Neddylation Blockade on Slug-Dependent Cancer Cell Migration Is Regulated by p53 Mutation Status
Abstract
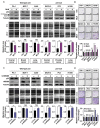
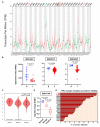
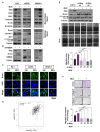
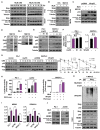
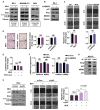
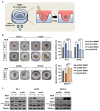
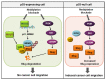
The tumor suppressor protein p53 is frequently inactivated in human malignancies, in which it is associated with cancer aggressiveness and metastasis. Because p53 is heavily involved in epithelial-mesenchymal transition (EMT), a primary step in cell migration, p53 regulation is important for preventing cancer metastasis. p53 function can be modulated by diverse post-translational modifications including neddylation, a reversible process that conjugates NEDD8 to target proteins and inhibits the transcriptional activity of p53. However, the role of p53 in cancer migration by neddylation has not been fully elucidated. In this study, we reported that neddylation blockade induces cell migration depending on p53 status, specifically via the EMT-promoting transcription factor Slug. In cancer cell lines expressing wild type p53, neddylation blockade increased the transcriptional activity of p53 and expression of its downstream genes p21 and MDM2, eventually promoting proteasomal degradation of Slug. In the absence of p53, neddylation blockade increased cell migration by activating the PI3K/Akt/mTOR/Slug signaling axis. Because mutant p53 was transcriptionally inactivated but maintained the ability to bind to Slug, neddylation blockade did not affect the migration of cells expressing mutant p53. Our findings highlight how the p53 expression status influences neddylation-mediated cell migration in multiple cancer cell lines via Slug.
Keywords: PI3K/Akt/mTOR signaling; Slug; epithelial-mesenchymal transition (EMT); migration; neddylation; p53.
Conflict of interest statement
The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Weiss L. Metastasis of cancer: A conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. 2000;19:214. - PubMed
-
- Powell E., Piwnica-Worms D., Piwnica-Worms H. Contribution of p53 to metastasis. Cancer Discov. 2014;4:405–414. doi: 10.1158/2159-8290.CD-13-0136. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

