Additive-manufactured Ti-6Al-4 V/Polyetheretherketone composite porous cage for Interbody fusion: bone growth and biocompatibility evaluation in a porcine model
- PMID: 33573634
- PMCID: PMC7879644
- DOI: 10.1186/s12891-021-04022-0
Additive-manufactured Ti-6Al-4 V/Polyetheretherketone composite porous cage for Interbody fusion: bone growth and biocompatibility evaluation in a porcine model
Abstract
Background: We developed a porous Ti alloy/PEEK composite interbody cage by utilizing the advantages of polyetheretherketone (PEEK) and titanium alloy (Ti alloy) in combination with additive manufacturing technology.
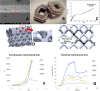
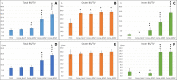
Methods: Porous Ti alloy/PEEK composite cages were manufactured using various controlled porosities. Anterior intervertebral lumbar fusion and posterior augmentation were performed at three vertebral levels on 20 female pigs. Each level was randomly implanted with one of the five cages that were tested: a commercialized pure PEEK cage, a Ti alloy/PEEK composite cage with nonporous Ti alloy endplates, and three composite cages with porosities of 40, 60, and 80%, respectively. Micro-computed tomography (CT), backscattered-electron SEM (BSE-SEM), and histological analyses were performed.
Results: Micro-CT and histological analyses revealed improved bone growth in high-porosity groups. Micro-CT and BSE-SEM demonstrated that structures with high porosities, especially 60 and 80%, facilitated more bone formation inside the implant but not outside the implant. Histological analysis also showed that bone formation was higher in Ti alloy groups than in the PEEK group.
Conclusion: The composite cage presents the biological advantages of Ti alloy porous endplates and the mechanical and radiographic advantages of the PEEK central core, which makes it suitable for use as a single implant for intervertebral fusion.
Keywords: 4 V (Ti alloy)/polyetheretherketone (PEEK) composite porous cage, porcine study; 6Al; Additive manufacturing (3D printing), Ti.
Conflict of interest statement
The authors have no financial competing interests related to this study. MHW is the associate editor of
Figures






Similar articles
-
Bony ingrowth potential of 3D-printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model.Spine J. 2018 Jul;18(7):1250-1260. doi: 10.1016/j.spinee.2018.02.018. Epub 2018 Feb 26. Spine J. 2018. PMID: 29496624 Free PMC article.
-
Comparison in the same intervertebral space between titanium-coated and uncoated PEEK cages in lumbar interbody fusion surgery.J Orthop Sci. 2020 Jul;25(4):565-570. doi: 10.1016/j.jos.2019.07.004. Epub 2019 Jul 30. J Orthop Sci. 2020. PMID: 31375363
-
Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: biomechanical, microcomputed tomographic, and histologic analyses.Spine J. 2017 Dec;17(12):1907-1916. doi: 10.1016/j.spinee.2017.06.034. Epub 2017 Jul 24. Spine J. 2017. PMID: 28751242
-
Clinical and radiological outcomes of titanium cage versus polyetheretherketone cage in lumbar interbody fusion: a systematic review and meta-analysis.Neurosurg Rev. 2025 Mar 12;48(1):295. doi: 10.1007/s10143-025-03453-w. Neurosurg Rev. 2025. PMID: 40075000
-
Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature.J Clin Neurosci. 2017 Oct;44:23-29. doi: 10.1016/j.jocn.2017.06.062. Epub 2017 Jul 21. J Clin Neurosci. 2017. PMID: 28736113 Review.
Cited by
-
The rational design, biofunctionalization and biological properties of orthopedic porous titanium implants: a review.Front Bioeng Biotechnol. 2025 Feb 26;13:1548675. doi: 10.3389/fbioe.2025.1548675. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40078794 Free PMC article. Review.
-
Definition, measurement, and function of pore structure dimensions of bioengineered porous bone tissue materials based on additive manufacturing: A review.Front Bioeng Biotechnol. 2023 Jan 4;10:1081548. doi: 10.3389/fbioe.2022.1081548. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36686223 Free PMC article. Review.
-
Evolution of polyetheretherketone (PEEK) and titanium interbody devices for spinal procedures: a comprehensive review of the literature.Eur Spine J. 2022 Oct;31(10):2547-2556. doi: 10.1007/s00586-022-07272-1. Epub 2022 Jun 10. Eur Spine J. 2022. PMID: 35689111 Review.
-
3D-printed polyether-ether-ketone/n-TiO2 composite enhances the cytocompatibility and osteogenic differentiation of MC3T3-E1 cells by downregulating miR-154-5p.Open Med (Wars). 2023 Jan 28;18(1):20230636. doi: 10.1515/med-2023-0636. eCollection 2023. Open Med (Wars). 2023. PMID: 36760721 Free PMC article.
-
Corrosion Resistance of 3D Printed Ti6Al4V Gyroid Lattices with Varying Porosity.Materials (Basel). 2022 Jul 9;15(14):4805. doi: 10.3390/ma15144805. Materials (Basel). 2022. PMID: 35888273 Free PMC article.
References
-
- Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, & Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine, 2019;44(5):369-76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

