SGLT-2 inhibitors and atrial fibrillation in the Food and Drug Administration adverse event reporting system
- PMID: 33573667
- PMCID: PMC7879696
- DOI: 10.1186/s12933-021-01243-4
SGLT-2 inhibitors and atrial fibrillation in the Food and Drug Administration adverse event reporting system
Abstract
Background: Sodium glucose cotransporter-2 inhibitors (SGLT2i) reduce the risk of heart failure and new data show they can prevent atrial fibrillation (AF). We examined the association between SGLT2i and AF in the Food and Drug Administration adverse event reporting system (FAERS).
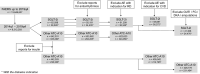
Methods: We mined the FAERS from 2014q1 to 2019q4 to compare AF reporting for SGLT-2 i versus reports for other glucose lowering medications (ATC10 class). Several exclusions were sequentially applied for: concomitant medications; diabetes, cardiovascular or renal disease indication; reports for competing adverse events (genitourinary tract infections, ketoacidosis, Fournier's gangrene, amputation). We provide descriptive statistics and calculated proportional reporting ratios (PRR).
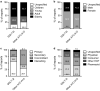
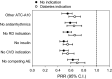
Results: There were 62,098 adverse event reports for SGLT2i and 642,031 reports for other ATC10 drugs. The reporting of AF was significantly lower with SGLT2i than with other ATC10 drugs (4.8 versus 8.7/1000; p < 0.001) with a PRR of 0.55 (0.49-0.62). Results did not change substantially after excluding reports listing insulin (PRR 0.49) or anti-arrhythmics (PRR 0.59) as suspect or concomitant drugs, excluding reports with indications for cardiovascular disease (PRR 0.49) or renal disease (PRR 0.55), and those filed for competing adverse events (PRR 0.63). Results were always statistically significant whether the diabetes indication was specified. Negative and positive controls confirmed internal validity of the database.
Conclusions: In a large pharmacovigilance database, AF was robustly and consistently reported more frequently for diabetes medications other than SGLT2i. This finding complements available evidence from trials supporting a protective role of SGLT2i against the occurrence of AF.
Keywords: Clinical practice; Complications; Epidemiology; Observational; Pharmacovigilance; Type 2 diabetes.
Conflict of interest statement
BMB received lecture or advisory board fees from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Mundipharma, Novartis, Novo Nordisk, and Sanofi. AA received research grants, lecture or advisory board fees from: Merck Sharp & Dome, AstraZeneca, Novartis, Boehringer-Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, and Takeda. GPF received grants, honoraria or lecture fees from Abbott, Astrazeneca, Boehringer, Lilly, Novo Nordisk, Sanofi. ER has no competing interests.
Figures
References
-
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. - DOI - PubMed
-
- Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141(15):1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

