Barriers to completing colonoscopy after a positive fecal occult blood test
- PMID: 33573698
- PMCID: PMC7879608
- DOI: 10.1186/s13584-021-00444-2
Barriers to completing colonoscopy after a positive fecal occult blood test
Abstract
Background: Colorectal cancer leads to significant morbidity and mortality. Early detection and treatment are essential. Screening using fecal occult blood tests has increased significantly, but adherence to colonoscopy follow-up is suboptimal, increasing CRC mortality risk. The aim of this study was to identify barriers to colonoscopy following a positive FOBT at the level of the patient, physician, organization and policymakers.
Methods: This mixed methods study was conducted at two health care organizations in Israel. The study included retrospective analyses of 45,281 50-74 year-old members with positive fecal immunochemical tests from 2010 to 2014, and a survey of 772 patients with a positive test during 2015, with and without follow-up. The qualitative part of the study included focus groups with primary physicians and gastroenterologists and in-depth interviews with opinion leaders in healthcare.
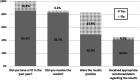
Results: Patient lack of comprehension regarding the test was the strongest predictor of non-adherence to follow-up. Older age, Arab ethnicity, and lower socio economic status significantly reduced adherence. We found no correlation with gender, marital status, patient activation, waiting time for appointments or distance from gastroenterology clinics. Primary care physicians underestimate non-adherence rates. They feel responsible for patient follow-up, but express lack of time and skills that will allow them to ensure adherence among their patients. Gastroenterologists do not consider fecal occult blood an effective tool for CRC detection, and believe that all patients should undergo colonoscopy. Opinion leaders in the healthcare field do not prioritize the issue of follow-up after a positive screening test for colorectal cancer, although they understand the importance.
Conclusions: We identified important barriers that need to be addressed to improve the effectiveness of the screening program. Targeted interventions for populations at risk for non-adherence, specifically for those with low literacy levels, and better explanation of the need for follow-up as a routine need to be set in place. Lack of agreement between screening recommendations and gastroenterologist opinion, and lack of awareness among healthcare authority figures negatively impact the screening program need to be addressed at the organizational and national level.
Trial registration: This study was approved by the IRB in both participating organizations (Meuhedet Health Care Institutional Review Board #02-2-5-15, Maccabi Healthcare Institutional Review Board BBI-0025-16). Participant consent was waived by both IRB's.
Keywords: Adherence; Cancer; Colonoscopy; Positive colorectal cancer screening.
Conflict of interest statement
None of the authors have competing interests.
Figures
References
-
- Anttila A, Lönnberg S, Ponti A, Suonio E, Villain P, Coebergh JW, von Karsa L. Towards better implementation of cancer screening in Europe through improved monitoring and evaluation and greater engagement of cancer registries. Eur J Cancer. 2015;51(9):1080–1081. doi: 10.1016/j.ejca.2015.05.001. - DOI - PubMed
-
- Colorectal Cancer: Screening, release date: June 2016 U.S. Preventive Task Force,. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummar.... Accessed 20 February 2020.
-
- Hagoel L. Colorectal Cancer- early detection. 2012.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

