Elevated cAMP Protects against Diclofenac-Induced Toxicity in Primary Rat Hepatocytes: A Protective Effect Mediated by the Exchange Protein Directly Activated by cAMP/cAMP-Regulated Guanine Nucleotide Exchange Factors
- PMID: 33574047
- PMCID: PMC11033960
- DOI: 10.1124/molpharm.120.000217
Elevated cAMP Protects against Diclofenac-Induced Toxicity in Primary Rat Hepatocytes: A Protective Effect Mediated by the Exchange Protein Directly Activated by cAMP/cAMP-Regulated Guanine Nucleotide Exchange Factors
Abstract
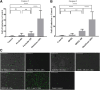
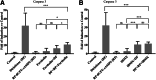
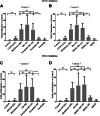
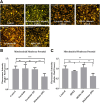
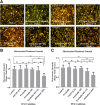
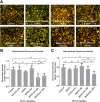
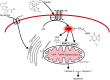
Chronic consumption of the nonsteroidal anti-inflammatory drug diclofenac may induce drug-induced liver injury (DILI). The mechanism of diclofenac-induced liver injury is partially elucidated and involves mitochondrial damage. Elevated cAMP protects hepatocytes against bile acid-induced injury. However, it is unknown whether cAMP protects against DILI and, if so, which downstream targets of cAMP are implicated in the protective mechanism, including the classic protein kinase A (PKA) pathway or alternative pathways like the exchange protein directly activated by cAMP (EPAC). The aim of this study was to investigate whether cAMP and/or its downstream targets protect against diclofenac-induced injury in hepatocytes. Rat hepatocytes were exposed to 400 µmol/l diclofenac. Apoptosis and necrosis were measured by caspase-3 activity assay and Sytox green staining, respectively. Mitochondrial membrane potential (MMP) was measured by JC-10 staining. mRNA and protein expression were assessed by quantitative polymerase chain reaction (qPCR) and Western blot, respectively. The cAMP-elevating agent 7β-acetoxy-8,13-epoxy-1α,6β,9α-trihydroxylabd-14-en-11-one (forskolin), the pan-phosphodiesterase inhibitor IBMX, and EPAC inhibitors 5,7-dibromo-6-fluoro-3,4-dihydro-2-methyl-1(2H)-quinoline carboxaldehyde (CE3F4) and ESI-O5 were used to assess the role of cAMP and its effectors, PKA or EPAC. Diclofenac exposure induced apoptotic cell death and loss of MMP in hepatocytes. Both forskolin and IBMX prevented diclofenac-induced apoptosis. EPAC inhibition but not PKA inhibition abolished the protective effect of forskolin and IBMX. Forskolin and IBMX preserved the MMP, whereas both EPAC inhibitors diminished this effect. Both EPAC1 and EPAC2 were expressed in hepatocytes and localized in mitochondria. cAMP elevation protects hepatocytes against diclofenac-induced cell death, a process primarily involving EPACs. The cAMP/EPAC pathway may be a novel target for treatment of DILI. SIGNIFICANCE STATEMENT: This study shows two main highlights. First, elevated cAMP levels protect against diclofenac-induced apoptosis in primary hepatocytes via maintenance of mitochondrial integrity. In addition, this study proposes the existence of mitochondrial cAMP-EPAC microdomains in rat hepatocytes, opening new avenues for targeted therapy in drug-induced liver injury (DILI). Both EPAC1 and EPAC2, but not protein kinase A, are responsible for this protective effect. Our findings present cAMP-EPAC as a potential target for the treatment of DILI and liver injury involving mitochondrial dysfunction.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare that they have no conflict of interest with other people or organizations during the preparation of this work.
Figures



Similar articles
-
Metformin protects against diclofenac-induced toxicity in primary rat hepatocytes by preserving mitochondrial integrity via a pathway involving EPAC.Biomed Pharmacother. 2021 Nov;143:112072. doi: 10.1016/j.biopha.2021.112072. Epub 2021 Aug 28. Biomed Pharmacother. 2021. PMID: 34464747
-
Introduction of aromatic ring-containing substituents in cyclic nucleotides is associated with inhibition of toxin uptake by the hepatocyte transporters OATP 1B1 and 1B3.PLoS One. 2014 Apr 16;9(4):e94926. doi: 10.1371/journal.pone.0094926. eCollection 2014. PLoS One. 2014. PMID: 24740327 Free PMC article.
-
PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways.Cell Signal. 2008 May;20(5):803-14. doi: 10.1016/j.cellsig.2007.12.011. Epub 2007 Dec 28. Cell Signal. 2008. PMID: 18276108
-
The future of EPAC-targeted therapies: agonism versus antagonism.Trends Pharmacol Sci. 2015 Apr;36(4):203-14. doi: 10.1016/j.tips.2015.02.003. Epub 2015 Mar 3. Trends Pharmacol Sci. 2015. PMID: 25744542 Free PMC article. Review.
-
Cell physiology of cAMP sensor Epac.J Physiol. 2006 Nov 15;577(Pt 1):5-15. doi: 10.1113/jphysiol.2006.119644. Epub 2006 Sep 14. J Physiol. 2006. PMID: 16973695 Free PMC article. Review.
Cited by
-
Thymoquinone mitigates diclofenac-induced hepatorenal toxicity in male Wistar rats by balancing the redox state and modulating Bax/Bcl-2/caspase-3 apoptotic pathways and NF-κB signaling.Res Pharm Sci. 2025 Feb 20;20(1):95-108. doi: 10.4103/RPS.RPS_141_24. eCollection 2025 Feb. Res Pharm Sci. 2025. PMID: 40190828 Free PMC article.
-
The role of exosomal lncRNAs in acetaminophen-induced induced liver injury in SD rats.Noncoding RNA Res. 2024 May 23;9(4):1190-1202. doi: 10.1016/j.ncrna.2024.05.011. eCollection 2024 Dec. Noncoding RNA Res. 2024. PMID: 39026604 Free PMC article.
-
Morin Attenuates Diclofenac-Induced Hepatocellular Death Injury via Nrf2/Ho-1/NQO1, Beclin-1/LC3A/LC3B and p53/Bax/Caspase Signalling Pathways.J Biochem Mol Toxicol. 2025 Jul;39(7):e70372. doi: 10.1002/jbt.70372. J Biochem Mol Toxicol. 2025. PMID: 40551516 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

