Molecular principles of Piwi-mediated cotranscriptional silencing through the dimeric SFiNX complex
- PMID: 33574069
- PMCID: PMC7919418
- DOI: 10.1101/gad.347989.120
Molecular principles of Piwi-mediated cotranscriptional silencing through the dimeric SFiNX complex
Abstract
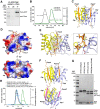
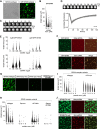
Nuclear Argonaute proteins, guided by their bound small RNAs to nascent target transcripts, mediate cotranscriptional silencing of transposons and repetitive genomic loci through heterochromatin formation. The molecular mechanisms involved in this process are incompletely understood. Here, we show that the SFiNX complex, a silencing mediator downstream from nuclear Piwi-piRNA complexes in Drosophila, facilitates cotranscriptional silencing as a homodimer. The dynein light chain protein Cut up/LC8 mediates SFiNX dimerization, and its function can be bypassed by a heterologous dimerization domain, arguing for a constitutive SFiNX dimer. Dimeric, but not monomeric SFiNX, is capable of forming molecular condensates in a nucleic acid-stimulated manner. Mutations that prevent SFiNX dimerization result in loss of condensate formation in vitro and the inability of Piwi to initiate heterochromatin formation and silence transposons in vivo. We propose that multivalent SFiNX-nucleic acid interactions are critical for heterochromatin establishment at piRNA target loci in a cotranscriptional manner.
Keywords: Drosophila oogenesis; LC8; Panoramix; Piwi; cotranscriptional silencing; heterochromatin formation; molecular condensates; piRNA pathway; transposon silencing.
© 2021 Schnabl et al.; Published by Cold Spring Harbor Laboratory Press.
Figures





References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954. 10.1107/S0907444902016657 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
