Rapid Depletion of Intratumoral Regulatory T Cells Induces Synchronized CD8 T- and NK-cell Activation and IFNγ-Dependent Tumor Vessel Regression
- PMID: 33574087
- PMCID: PMC8178213
- DOI: 10.1158/0008-5472.CAN-20-2673
Rapid Depletion of Intratumoral Regulatory T Cells Induces Synchronized CD8 T- and NK-cell Activation and IFNγ-Dependent Tumor Vessel Regression
Abstract
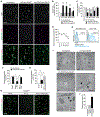
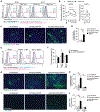
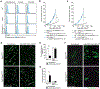
Regulatory T cells (Tregs) are known to inhibit antitumor immunity, yet the specific mechanism by which intratumoral Tregs promote tumor growth remains unclear. To better understand the roles of intratumoral Tregs, we selectively depleted tumor-infiltrating Tregs using anti-CD25-F(ab')2 near-infrared photoimmunotherapy. Depletion of tumor-infiltrating Tregs induced transient but synchronized IFNγ expression in CD8 T and natural killer (NK) cells. Despite the small fraction of CD8 T and NK cells contained within examined tumors, IFNγ produced by these CD8 T and NK cells led to efficient and rapid tumor vessel regression, intratumoral ischemia, and tumor necrosis/apoptosis and growth suppression. IFNγ receptor expression on vascular endothelial cells was required for these effects. Similar findings were observed in the early phase of systemic Treg depletion in tumor-bearing Foxp3DTR mice; combination with IL15 therapy further inhibited tumor growth and achieved increased complete regression. These results indicate the pivotal roles of intratumoral Tregs in maintaining tumor vessels and tumor growth by suppressing CD8 T and NK cells from producing IFNγ, providing insight into the mechanism of Treg-targeting therapies. SIGNIFICANCE: Intratumoral Treg depletion induces synchronized intratumoral CD8 T- and NK-cell activation, IFNγ-dependent tumor vessel regression, and ischemic tumor necrosis/apoptosis, indicating the roles of intratumoral Tregs to support the tumor vasculature. GRAPHICAL ABSTRACT: http://cancerres.aacrjournals.org/content/canres/81/11/3092/F1.large.jpg.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest statement
P.L.C., H.K., and N.S. have filed U.S. national and international patent applications for near infrared photoimmunotherapy of suppressor cells to treat cancer used in this study.
Regarding C.O.: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and 75N910D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Figures




Similar articles
-
Mouse cytomegalovirus infection overrules T regulatory cell suppression on natural killer cells.Virol J. 2014 Aug 9;11:145. doi: 10.1186/1743-422X-11-145. Virol J. 2014. PMID: 25108672 Free PMC article.
-
Therapeutic depletion of CCR8+ tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy.J Immunother Cancer. 2021 Feb;9(2):e001749. doi: 10.1136/jitc-2020-001749. J Immunother Cancer. 2021. PMID: 33589525 Free PMC article.
-
Combination of SLC administration and Tregs depletion is an attractive strategy for targeting hepatocellular carcinoma.Mol Cancer. 2013 Dec 5;12(1):153. doi: 10.1186/1476-4598-12-153. Mol Cancer. 2013. PMID: 24304581 Free PMC article.
-
Impact of dietary components on NK and Treg cell function for cancer prevention.Mol Carcinog. 2015 Sep;54(9):669-78. doi: 10.1002/mc.22301. Epub 2015 Apr 1. Mol Carcinog. 2015. PMID: 25845339 Free PMC article. Review.
-
The FOX transcription factors regulate vascular pathology, diabetes and Tregs.Front Biosci (Schol Ed). 2009 Jun 1;1(2):420-36. doi: 10.2741/S35. Front Biosci (Schol Ed). 2009. PMID: 19482711 Free PMC article. Review.
Cited by
-
A Comprehensive Analysis of HAVCR1 as a Prognostic and Diagnostic Marker for Pan-Cancer.Front Genet. 2022 Jun 8;13:904114. doi: 10.3389/fgene.2022.904114. eCollection 2022. Front Genet. 2022. PMID: 35754803 Free PMC article.
-
PD-L1 near Infrared Photoimmunotherapy of Ovarian Cancer Model.Cancers (Basel). 2022 Jan 26;14(3):619. doi: 10.3390/cancers14030619. Cancers (Basel). 2022. PMID: 35158887 Free PMC article.
-
A novel CD47-blocking peptide fused to pro-apoptotic KLA repeat inhibits lung cancer growth in mice.Cancer Immunol Immunother. 2023 Dec;72(12):4179-4194. doi: 10.1007/s00262-023-03554-9. Epub 2023 Oct 13. Cancer Immunol Immunother. 2023. PMID: 37831145 Free PMC article.
-
Prospects and applications of NK therapy in the treatment of gliomas (Review).Oncol Rep. 2025 Aug;54(2):88. doi: 10.3892/or.2025.8921. Epub 2025 Jun 6. Oncol Rep. 2025. PMID: 40476557 Free PMC article. Review.
-
Alleviating hypoxia to improve cancer immunotherapy.Oncogene. 2023 Dec;42(49):3591-3604. doi: 10.1038/s41388-023-02869-2. Epub 2023 Oct 26. Oncogene. 2023. PMID: 37884747 Review.
References
-
- Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol 2016;16:599–611 - PubMed
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine 2004;10:942–9 - PubMed
-
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer 2012;12:298–306 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

