Detection of involved margins in breast specimens with X-ray phase-contrast computed tomography
- PMID: 33574584
- PMCID: PMC7878478
- DOI: 10.1038/s41598-021-83330-w
Detection of involved margins in breast specimens with X-ray phase-contrast computed tomography
Abstract
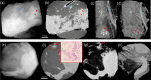
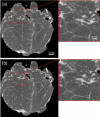
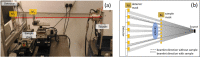
Margins of wide local excisions in breast conserving surgery are tested through histology, which can delay results by days and lead to second operations. Detection of margin involvement intraoperatively would allow the removal of additional tissue during the same intervention. X-ray phase contrast imaging (XPCI) provides soft tissue sensitivity superior to conventional X-rays: we propose its use to detect margin involvement intraoperatively. We have developed a system that can perform phase-based computed tomography (CT) scans in minutes, used it to image 101 specimens approximately half of which contained neoplastic lesions, and compared results against those of a commercial system. Histological analysis was carried out on all specimens and used as the gold standard. XPCI-CT showed higher sensitivity (83%, 95% CI 69-92%) than conventional specimen imaging (32%, 95% CI 20-49%) for detection of lesions at margin, and comparable specificity (83%, 95% CI 70-92% vs 86%, 95% CI 73-93%). Within the limits of this study, in particular that specimens obtained from surplus tissue typically contain small lesions which makes detection more difficult for both methods, we believe it likely that the observed increase in sensitivity will lead to a comparable reduction in the number of re-operations.
Conflict of interest statement
Hawker, Smit, Astolfo, Larkin, Haig and Bate are, or were at the time the research was carried out, Nikon employees. Waltham is a Nikon consultant. Endrizzi, Munro and Olivo are named inventors on patents owned by UCL protecting the technology used to obtain the described results. All other authors have no competing/conflicts of interest.
Figures


References
-
- Early and locally advanced breast cancer: diagnosis and management. NICE 2018 Recommendation, NG101 NICE Guidance, UK. https://www.nice.org.uk/guidance/ng101/chapter/Recommendations. Accessed June 2019.
-
- Tang SS-K, et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur. J. Cancer. 2017;84:315–324. doi: 10.1016/j.ejca.2017.07.032. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

