Evaluation of Anti-Biofilm Capability of Cordycepin Against Candida albicans
- PMID: 33574683
- PMCID: PMC7872900
- DOI: 10.2147/IDR.S285690
Evaluation of Anti-Biofilm Capability of Cordycepin Against Candida albicans
Abstract
Introduction: The opportunistic pathogen Candida albicans can form biofilms, resulting in drug resistance with great risk to medical treatment.
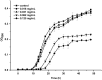
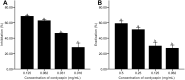
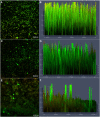
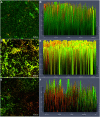
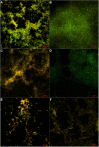
Methodology: We investigated the ability of C. albicans to form biofilms on different materials, as well as the inhibitory and eradicating effects of cordycepin on biofilm. The action mechanism of cordycepin against biofilm was studied by crystal violet staining, XTT [2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] reduction method, phenol-sulfuric acid method, cellular superficial hydrophobicity (CSH) assay, and confocal laser scanning microscope observation. We also evaluated the acute toxicity of cordycepin in vivo.
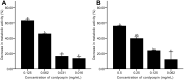
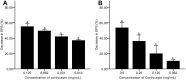
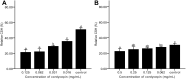
Results: The results showed facile formation of biofilms by C. albicans on polypropylene. The 50% minimum inhibitory concentration (MIC50) of cordycepin was 0.062 mg/mL. A concentration of 0.125 mg/mL significantly decreased biofilm formation, metabolic activity, secretion of extracellular polysaccharides, and relative CSH. Cordycepin could inhibit biofilm formation at low concentration without affecting fungal growth. In addition, cordycepin effectively eradicated 59.14% of mature biofilms of C. albicans at a concentration of 0.5 mg/mL. For acute toxicity, the LD50 (50% of lethal dose) of cordycepin was determined as higher than 500 mg/kg for mice.
Conclusion: The results of this study show that cordycepin significantly inhibited and eradicated biofilms by decreasing metabolic activity, the ratio of living cells, the hydrophobicity, and damaging the extracellular polysaccharides of biofilm. These findings should facilitate more effective application of cordycepin and suggest a new direction for the treatment of fungal infections.
Keywords: Candida albicans; biofilm; cordycepin; eradication; inhibition.
© 2021 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
In vitro efficacy of eugenol in inhibiting single and mixed-biofilms of drug-resistant strains of Candida albicans and Streptococcus mutans.Phytomedicine. 2019 Feb 15;54:206-213. doi: 10.1016/j.phymed.2018.10.005. Epub 2018 Oct 9. Phytomedicine. 2019. PMID: 30668370
-
Antibacterial activity of food-grade chitosan against Vibrio parahaemolyticus biofilms.Microb Pathog. 2017 Sep;110:291-297. doi: 10.1016/j.micpath.2017.07.011. Epub 2017 Jul 11. Microb Pathog. 2017. PMID: 28710011
-
In Vitro Effects of Plantago Major Extract, Aucubin, and Baicalein on Candida albicans Biofilm Formation, Metabolic Activity, and Cell Surface Hydrophobicity.J Prosthodont. 2017 Aug;26(6):508-515. doi: 10.1111/jopr.12411. Epub 2015 Nov 30. J Prosthodont. 2017. PMID: 26618515
-
Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida albicans.Front Cell Infect Microbiol. 2017 Oct 16;7:447. doi: 10.3389/fcimb.2017.00447. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29085811 Free PMC article.
-
Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: An in vitro study.J Prosthet Dent. 2021 Apr;125(4):707.e1-707.e6. doi: 10.1016/j.prosdent.2020.12.017. Epub 2021 Jan 16. J Prosthet Dent. 2021. PMID: 33468317
Cited by
-
Antibiofilm Activity of Essential Fatty Acids Against Candida albicans from Vulvovaginal Candidiasis and Bloodstream Infections.Infect Drug Resist. 2022 Aug 3;15:4181-4193. doi: 10.2147/IDR.S373991. eCollection 2022. Infect Drug Resist. 2022. PMID: 35946033 Free PMC article.
-
Lycosin-II Exhibits Antifungal Activity and Inhibits Dual-Species Biofilm by Candida albicans and Staphylococcus aureus.J Fungi (Basel). 2022 Aug 24;8(9):901. doi: 10.3390/jof8090901. J Fungi (Basel). 2022. PMID: 36135626 Free PMC article.
-
Antimicrobial and antibiofilm effects of essential fatty acids against clinically isolated vancomycin-resistant Enterococcus faecium.Front Cell Infect Microbiol. 2023 Sep 29;13:1266674. doi: 10.3389/fcimb.2023.1266674. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37842001 Free PMC article.
-
Antifungal, anti-biofilm, and anti-hyphal properties of N-substituted phthalimide derivatives against Candida species.Front Cell Infect Microbiol. 2024 Jun 5;14:1414618. doi: 10.3389/fcimb.2024.1414618. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38903941 Free PMC article.
-
Quinic acid: a potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa.Chin Med. 2021 Aug 6;16(1):72. doi: 10.1186/s13020-021-00481-8. Chin Med. 2021. PMID: 34362401 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

