Genetic and Epigenetic Causes of Pituitary Adenomas
- PMID: 33574795
- PMCID: PMC7870789
- DOI: 10.3389/fendo.2020.596554
Genetic and Epigenetic Causes of Pituitary Adenomas
Abstract
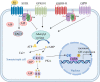
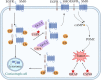
Pituitary adenomas (PAs) can be classified as non-secreting adenomas, somatotroph adenomas, corticotroph adenomas, lactotroph adenomas, and thyrotroph adenomas. Substantial advances have been made in our knowledge of the pathobiology of PAs. To obtain a comprehensive understanding of the molecular biological characteristics of different types of PAs, we reviewed the important advances that have been made involving genetic and epigenetic variation, comprising genetic mutations, chromosome number variations, DNA methylation, microRNA regulation, and transcription factor regulation. Classical tumor predisposition syndromes include multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) syndromes, Carney complex, and X-LAG syndromes. PAs have also been described in association with succinate dehydrogenase-related familial PA, neurofibromatosis type 1, and von Hippel-Lindau, DICER1, and Lynch syndromes. Patients with aryl hydrocarbon receptor-interacting protein (AIP) mutations often present with pituitary gigantism, either in familial or sporadic adenomas. In contrast, guanine nucleotide-binding protein G(s) subunit alpha (GNAS) and G protein-coupled receptor 101 (GPR101) mutations can lead to excess growth hormone. Moreover, the deubiquitinase gene USP8, USP48, and BRAF mutations are associated with adrenocorticotropic hormone production. In this review, we describe the genetic and epigenetic landscape of PAs and summarize novel insights into the regulation of pituitary tumorigenesis.
Keywords: Cushing’s disease; acromegaly; molecular markers; non-secreting adenomas; pituitary adenomas.
Copyright © 2021 Chang, Yang, Bao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Clinical and Molecular Update on Genetic Causes of Pituitary Adenomas.Horm Metab Res. 2020 Aug;52(8):553-561. doi: 10.1055/a-1143-5930. Epub 2020 Apr 16. Horm Metab Res. 2020. PMID: 32299111 Review.
-
Novel Genetic Causes of Pituitary Adenomas.Clin Cancer Res. 2016 Oct 15;22(20):5030-5042. doi: 10.1158/1078-0432.CCR-16-0452. Clin Cancer Res. 2016. PMID: 27742789 Review.
-
Screening for genetic causes of growth hormone hypersecretion.Growth Horm IGF Res. 2016 Oct-Dec;30-31:52-57. doi: 10.1016/j.ghir.2016.10.004. Epub 2016 Oct 12. Growth Horm IGF Res. 2016. PMID: 27756606 Review.
-
Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients.J Clin Endocrinol Metab. 2012 Apr;97(4):E663-70. doi: 10.1210/jc.2011-2291. Epub 2012 Feb 8. J Clin Endocrinol Metab. 2012. PMID: 22319033
-
The Genetics of Pituitary Adenomas.J Clin Med. 2019 Dec 21;9(1):30. doi: 10.3390/jcm9010030. J Clin Med. 2019. PMID: 31877737 Free PMC article. Review.
Cited by
-
Somatic GNAS mutations in acromegaly: prevalence, clinical features and gender differences.Endocr Connect. 2024 Dec 20;14(1):e240266. doi: 10.1530/EC-24-0266. Print 2025 Jan 1. Endocr Connect. 2024. PMID: 39513543 Free PMC article.
-
[Unification of pathomorphological examination of patients with neuroendocrine tumors of the pituitary gland. Controversial issues of the new classification].Probl Endokrinol (Mosk). 2023 Nov 14;70(3):31-45. doi: 10.14341/probl13376. Probl Endokrinol (Mosk). 2023. PMID: 39069771 Free PMC article. Review. Russian.
-
Pathogenesis, clinical features, and treatment of plurihormonal pituitary adenoma.Front Neurosci. 2024 Jan 8;17:1323883. doi: 10.3389/fnins.2023.1323883. eCollection 2023. Front Neurosci. 2024. PMID: 38260014 Free PMC article. Review.
-
Emerging Roles of miRNA, lncRNA, circRNA, and Their Cross-Talk in Pituitary Adenoma.Cells. 2022 Sep 19;11(18):2920. doi: 10.3390/cells11182920. Cells. 2022. PMID: 36139495 Free PMC article. Review.
-
RETRACTED: The Emerging Role of Epigenetics in Metabolism and Endocrinology.Biology (Basel). 2023 Feb 6;12(2):256. doi: 10.3390/biology12020256. Biology (Basel). 2023. Retraction in: Biology (Basel). 2025 Jun 16;14(6):705. doi: 10.3390/biology14060705. PMID: 36829533 Free PMC article. Retracted. Review.
References
-
- Salomon MP, Wang X, Marzese DM, Hsu SC, Nelson N, Zhang X, et al. The Epigenomic Landscape of Pituitary Adenomas Reveals Specific Alterations and Differentiates Among Acromegaly, Cushing’s Disease and Endocrine-Inactive Subtypes. Clin Cancer Res (2018) 24(17):4126–36. 10.1158/1078-0432.ccr-17-2206 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

