Experimental Trypanosoma cruzi Infection Induces Pain in Mice Dependent on Early Spinal Cord Glial Cells and NFκB Activation and Cytokine Production
- PMID: 33574810
- PMCID: PMC7870690
- DOI: 10.3389/fimmu.2020.539086
Experimental Trypanosoma cruzi Infection Induces Pain in Mice Dependent on Early Spinal Cord Glial Cells and NFκB Activation and Cytokine Production
Abstract
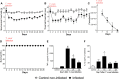
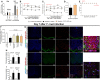
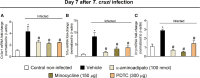
The neglected tropical infirmity Chagas disease (CD) presents high mortality. Its etiological agent T. cruzi is transmitted by infected hematophagous insects. Symptoms of the acute phase of the infection include fever, fatigue, body aches, and headache, making diagnosis difficult as they are present in other illnesses as well. Thus, in endemic areas, individuals with undetermined pain may be considered for CD. Although pain is a characteristic symptom of CD, its cellular and molecular mechanisms are unknown except for demonstration of a role for peripheral TNF-α in CD pain. In this study, we evaluate the role of spinal cord glial cells in experimental T. cruzi infection in the context of pain using C57BL/6 mice. Pain, parasitemia, survival, and glial and neuronal function as well as NFκB activation and cytokine/chemokine production were assessed. T. cruzi infection induced chronic mechanical and thermal hyperalgesia. Systemic TNF-α and IL-1β peaked 14 days postinfection (p.i.). Infected mice presented increased spinal gliosis and NFκB activation compared to uninfected mice at 7 days p.i. Glial and NFκB inhibitors limited T. cruzi-induced pain. Nuclear phosphorylated NFκB was detected surrounded by glia markers, and glial inhibitors reduced its detection. T. cruzi-induced spinal cord production of cytokines/chemokines was also diminished by glial inhibitors. Dorsal root ganglia (DRG) neurons presented increased activity in infected mice, and the production of inflammatory mediators was counteracted by glial/NFκB inhibitors. The present study unveils the contribution of DRG and spinal cord cellular and molecular events leading to pain in T. cruzi infection, contributing to a better understanding of CD pathology.
Keywords: NFκB; Trypanosoma cruzi; cytokine; glial cells; pain.
Copyright © 2021 Borghi, Fattori, Carvalho, Tatakihara, Zaninelli, Pinho-Ribeiro, Ferraz, Staurengo-Ferrari, Casagrande, Pavanelli, Cunha, Cunha, Pinge-Filho and Verri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


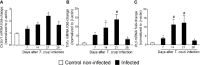



References
-
- Silva JS, Vespa GN, Cardoso MA, Aliberti JC, Cunha FQ. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun (1995) 63(12):4862–7. 10.1128/IAI.63.12.4862-4867.1995 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

