The Inhibitory Effect of Curosurf® and Alveofact® on the Formation of Neutrophil Extracellular Traps
- PMID: 33574811
- PMCID: PMC7871907
- DOI: 10.3389/fimmu.2020.582895
The Inhibitory Effect of Curosurf® and Alveofact® on the Formation of Neutrophil Extracellular Traps
Abstract
Background: Neutrophil extracellular traps (NETs) are a defense mechanism in which neutrophils cast a net-like structure in response to microbial infection. NETs consist of decondensed chromatin and about 30 enzymes and peptides. Some components, such as neutrophil elastase (NE) and myeloperoxidase (MPO), present antimicrobial but also cytotoxic properties, leading to tissue injury. Many inflammatory diseases are associated with NETs, and their final role has not been identified. Pulmonary surfactant is known to have immunoregulatory abilities that alter the function of adaptive and innate immune cells. The aim of this study was to investigate the hypothesis that natural surfactant preparations inhibit the formation of NETs.
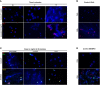
Methods: The effect of two natural surfactants (Alveofact® and Curosurf®) on spontaneous and phorbol-12-myristate-13-acetate-induced NET formation by neutrophils isolated by magnetic cell sorting from healthy individuals was examined. NETs were quantitatively detected by absorption and fluorometric-based assays for the NET-specific proteins (NE, MPO) and cell-free DNA. Immunofluorescence microscopy images were used for visualization.
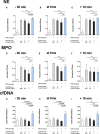
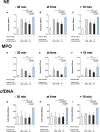
Results: Both surfactant preparations exerted a dose-dependent inhibitory effect on NET formation. Samples treated with higher concentrations and with 30 min pre-incubation prior to stimulation with phorbol-12-myristate-13-acetate had significantly lower levels of NET-specific proteins and cell-free DNA compared to untreated samples. Immunofluorescence microscopy confirmed these findings.
Conclusions: The described dose-dependent modulation of NET formation ex vivo suggests an interaction between exogenous surfactant supplementation and neutrophil granulocytes. The immunoregulatory effects of surfactant preparations should be considered for further examination of inflammatory diseases.
Keywords: alveofact®; anti-inflammatory; curosurf®; neutrophil extracellular traps; neutrophil granulocytes; pulmonary surfactant.
Copyright © 2021 Schulz, Pagerols Raluy, Kolman, Königs, Trochimiuk, Appl, Reinshagen, Boettcher and Trah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
NET Release of Long-Term Surviving Neutrophils.Front Immunol. 2022 Feb 15;13:815412. doi: 10.3389/fimmu.2022.815412. eCollection 2022. Front Immunol. 2022. PMID: 35242132 Free PMC article.
-
Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis.J Thromb Haemost. 2016 Mar;14(3):551-8. doi: 10.1111/jth.13239. Epub 2016 Feb 5. J Thromb Haemost. 2016. PMID: 26712312 Free PMC article.
-
CPHEN-017: Comprehensive phenotyping of neutrophil extracellular traps (NETs) on peripheral human neutrophils.Cytometry A. 2024 Aug;105(8):639-652. doi: 10.1002/cyto.a.24851. Epub 2024 Jun 12. Cytometry A. 2024. PMID: 38867433
-
The pro-tumor effect and the anti-tumor effect of neutrophils extracellular traps.Biosci Trends. 2020 Jan 20;13(6):469-475. doi: 10.5582/bst.2019.01326. Epub 2019 Dec 21. Biosci Trends. 2020. PMID: 31866615 Review.
-
Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing?Front Immunol. 2016 Aug 15;7:311. doi: 10.3389/fimmu.2016.00311. eCollection 2016. Front Immunol. 2016. PMID: 27574522 Free PMC article. Review.
Cited by
-
Exposure to Aldehyde Cherry e-Liquid Flavoring and Its Vaping Byproduct Disrupt Pulmonary Surfactant Biophysical Function.Environ Sci Technol. 2024 Jan 23;58(3):1495-1508. doi: 10.1021/acs.est.3c07874. Epub 2024 Jan 8. Environ Sci Technol. 2024. PMID: 38186267 Free PMC article.
-
Early Immunomodulatory Effects of Different Natural Surfactant Preparations in Preterms With Respiratory Distress.Front Pediatr. 2022 Mar 18;10:845780. doi: 10.3389/fped.2022.845780. eCollection 2022. Front Pediatr. 2022. PMID: 35372166 Free PMC article.
-
Neutrophil Extracellular Trap-Driven Occlusive Diseases.Cells. 2021 Aug 26;10(9):2208. doi: 10.3390/cells10092208. Cells. 2021. PMID: 34571857 Free PMC article. Review.
-
NET Release of Long-Term Surviving Neutrophils.Front Immunol. 2022 Feb 15;13:815412. doi: 10.3389/fimmu.2022.815412. eCollection 2022. Front Immunol. 2022. PMID: 35242132 Free PMC article.
-
In Vitro Dissolution and Permeability Testing of Inhalation Products: Challenges and Advances.Pharmaceutics. 2023 Mar 18;15(3):983. doi: 10.3390/pharmaceutics15030983. Pharmaceutics. 2023. PMID: 36986844 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

