Myeloid Ezh2 Deficiency Limits Atherosclerosis Development
- PMID: 33574814
- PMCID: PMC7871783
- DOI: 10.3389/fimmu.2020.594603
Myeloid Ezh2 Deficiency Limits Atherosclerosis Development
Abstract
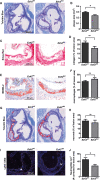
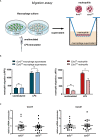
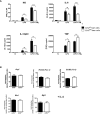
Macrophages define a key component of immune cells present in atherosclerotic lesions and are central regulators of the disease. Since epigenetic processes are important in controlling macrophage function, interfering with epigenetic pathways in macrophages might be a novel approach to combat atherosclerosis. Histone H3K27 trimethylation is a repressive histone mark catalyzed by polycomb repressive complex with EZH2 as the catalytic subunit. EZH2 is described to increase macrophage inflammatory responses by supressing the suppressor of cytokine signaling, Socs3. We previously showed that myeloid deletion of Kdm6b, an enzymes that in contrast to EZH2 removes repressive histone H3K27me3 marks, results in advanced atherosclerosis. Because of its opposing function and importance of EZH2 in macrophage inflammatory responses, we here studied the role of myeloid EZH2 in atherosclerosis. A myeloid-specific Ezh2 deficient mouse strain (Ezh2del) was generated (LysM-cre+ x Ezh2fl/fl) and bone marrow from Ezh2del or Ezh2wt mice was transplanted to Ldlr-/- mice which were fed a high fat diet for 9 weeks to study atherosclerosis. Atherosclerotic lesion size was significantly decreased in Ezh2del transplanted mice compared to control. The percentage of macrophages in the atherosclerotic lesion was similar, however neutrophil numbers were lower in Ezh2del transplanted mice. Correspondingly, the migratory capacity of neutrophils was decreased in Ezh2del mice. Moreover, peritoneal Ezh2del foam cells showed a reduction in the inflammatory response with reduced production of nitric oxide, IL-6 and IL-12. In Conclusion, myeloid Ezh2 deficiency impairs neutrophil migration and reduces macrophage foam cell inflammatory responses, both contributing to reduced atherosclerosis.
Keywords: H3K27; PRC2; atherosclerosis; epigenetic; histone modification; macrophage; polycomb.
Copyright © 2021 Neele, Chen, Gijbels, van der Velden, Hoeksema, Boshuizen, Van den Bossche, Tool, Matlung, van den Berg, Lutgens and de Winther.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

