Imaging the mitral valve: a primer for the interventional surgeon
- PMID: 33575173
- PMCID: PMC7867432
- DOI: 10.21037/acs-2020-mv-16
Imaging the mitral valve: a primer for the interventional surgeon
Abstract
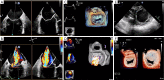
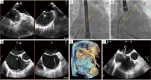
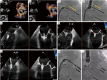
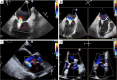
Transcatheter mitral valve interventions (TMVI) have evolved over the past decade as alternatives to open surgical repair for the therapeutic management of patients with severe mitral regurgitation (MR). Concurrent with the development of these technologies, quality multi-modality cardiac imaging has become essential in patient selection and procedural guidance. The former involves assessments of the pathophysiologic mechanisms of regurgitation, valvular anatomy and morphology, as well as objective quantification of the severity of MR. Both transthoracic and transesophageal echocardiography (TEE) are crucial and serve as the gateway to diagnosis and management of mitral valvular disease. Along with multi-detector computed tomography (CT) and cardiac magnetic resonance imaging (CMR), echocardiography plays an important role for preprocedural planning and evaluation of the spatial relationships of the mitral valvular complex with the coronary sinus, circumflex coronary artery and left ventricular (LV) outflow tract. Procedures that target mitral leaflets (e.g., MitraClip, PASCAL) or annulus (e.g., Cardioband, Carillon), or provide chordal (e.g., NeoChord, Harpoon) or valvular replacement, tend to be guided by TEE and assisted by fluoroscopy. As newer devices become available and outcomes of TMVI improve, cardiac imaging will undoubtedly continue to play an essential role in the success of percutaneous mitral valve repair (MVr) and replacement. The interventional surgeon of the future must therefore have a thorough understanding of the various imaging modalities while synthesizing and integrating novel concepts (e.g., neo-LV outflow tract) as applicable to assessing valvular function and pathology.
Keywords: Mitral regurgitation (MR); computed tomography (CT); interventional echocardiography; multimodality cardiac imaging; transcatheter mitral valve replacement (TMVR).
2021 Annals of Cardiothoracic Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: Dr. GHLT is a consultant for Medtronic, Abbott Structural Heart, and W. L. Gore & Associates. Dr. RPM reports the following financial interests/arrangements or affiliations: Steering Committee, Abbott TRILUMINATE Pivotal; Speakers Bureau, Edwards Lifesciences; Speakers Bureau, Medtronic Heart Valves; Medical Advisory Board, CardioCare-Edwards; Executive Committee, Medtronic TMVR, Officer and Stock Holder, Bay Labs-AI Company. The other authors have no conflicts of interest to declare.
Figures

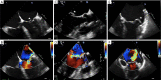
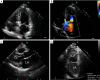
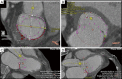
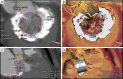


References
-
- Bonow RO, O'Gara PT, Adams DH, et al. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;75:2236-70. 10.1016/j.jacc.2020.02.005 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
